Scientific advancements like genomic editing have taken the world by storm, but have also blurred the lines between genetics and ethics. While this has been a growing phenomenon, in recent years, scientists have brought more attention to the non-coding region of DNA called “junk DNA.” Non-coding regions play key roles in regulating gene expression and chromatin structure. With gene editing, concerns rise, and many wonder if the intervention of these genomic regions is ethically okay for scientists to do. This is where this paper comes in to explore whether this is ethically justifiable to edit non-coding regions of DNA without fully
understanding its mechanism. An investigation of the CRISPR-babies case, together with therapeutic uses of gene editing, both can represent therapeutic breakthroughs, ethical concerns regarding consent, and the overall moral limits of scientific intervention.
What is Non-Coding DNA, and What Does It Do?
First off, non-coding DNA is the portion of the genome that does not code for an amino acid, thus leading to no creation of a protein. It instead works on gene expression by working through promoters, enhancers, and silencers that are all responsible for which genes turn on or off.1 In other cases, it can be transcribed into non-coding RNAs, which are involved in various cellular functions.2 The functions of these non-coding regions are not very clear, but the catalyst to these observations is all due to the completed Human Genome Project (2003), followed by the Encyclopedia of DNA Elements (ENCODE). Both aim to identify and characterize the functional elements of the human genome, including non-coding regions.3 These advancements led scientists to target specific genes, but it also led to unexpected consequences, known as compensatory effects.
Chromatin Accessibility and Gene Regulation
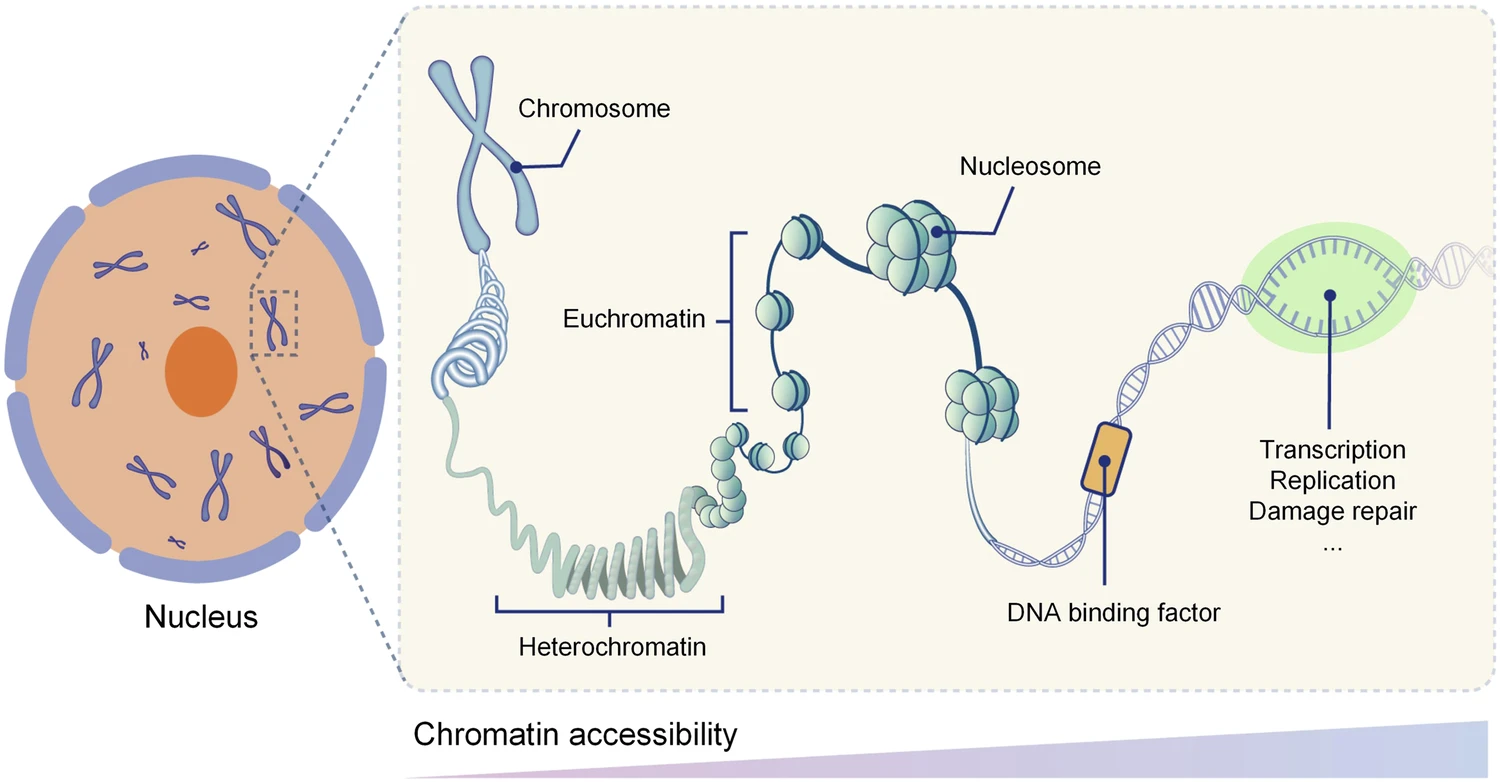
Recent ENCODE studies reveal that non-coding DNA is not as inactive as previously thought. Rather, it involves enhancer-promoter loops, chromatin accessibility, and RNA-binding interactions.3 A single enhancer can control multiple genes, and any minor edit in the non-coding region can affect this network, thus seen as a change that may appear silent. A “silent” change rarely shows phenotypes and creates uncertainty about the technical processes. A particular aspect of gene regulation involves chromatin accessibility, which is how tightly or loosely DNA is packed. Chromatin accessibility plays a crucial role in determining whether these enhancers or promoters are active. Regions of open chromatin are highly dynamic, changing across tissues and developmental stages, meaning that editing one non-coding region could have unintended consequences depending on when or where the edit occurs.
In fact, only about 2-3% of the human genome is accessible at any given time, and over 90% of these open sites are bound by transcription factors, which shows how vital and sensitive these areas are. Because of this, gene editing within these accessible non-coding regions is ethically complex: disrupting a single enhancer or promoter could alter entire gene networks that rely on the same open chromatin domain.5
Complexity and Development
Along the lines of gene editing, it is more complex and not necessarily straightforward. An example of this is seen in zebrafish when knocking out the egfl7 gene. The gene is responsible for the formation of blood vessel tubes, and when temporarily knocked down, it causes severe effects; however, during permanent genetic knockout using CRISPR-Cas9, it has no visible phenotypic effects.6 Such discrepancy may be linked to how chromatin accessibility changes during development and that certain regulatory regions may only be accessible during specific time windows, meaning a temporary knockout can interrupt crucial periods of gene activity that a permanent edit may miss.5 Another example involves transposable elements, known as “jumping genes,” which are sequences that can move throughout one’s genome. These elements are often harmless, but in some cases, they can disrupt gene function or regulation when inserted into non-coding regulatory regions. This leads to unintended effects and can complicate gene knockout studies.8
Gene Editing Technologies
CRISPR–Cas9 is known to be a fast and flexible tool for gene editing in cells and animal models.9 It is often used to introduce genetic alterations like insertions or deletions of a specific gene. Although there are no observed effects in permanent knockouts, there are times when researchers need temporary knockouts. This is where another genetic editing process comes in, known as the degron system. A common degron system is the Auxin-Inducible Degron (AID) system, which utilizes a plant auxin receptor to target a specific protein, degrading it temporarily.10
While both CRISPR and degron systems offer great advantages depending on the experimental goal, there is still the fear of choosing the wrong approach. Not fully understanding the genetic context leads to unintended consequences that can compromise an experiment or someone’s life. This raises the scientific and ethical concerns of off-target effects and long-term impacts. In fact, researchers have observed that off-target edits often occur in open chromatin regions, where the DNA is more physically exposed. Cas9 binding highlights the importance of mapping chromatin accessibility before any gene-editing attempt.5
Role in Therapeutics and Ethical Concerns
This brings us to a critical question of whether these ethical issues are outweighed by the impact these advancements have had. As of 2023, more than 200 people have been treated withexperimental CRISPR therapies. One patient by the name Victoria Gray advocates for gene therapy treatment since she has undergone the treatment herself. Gray has been an ongoing fighter of sickle-cell and underwent genetic editing treatment, which has turned her life around.12 Gray is one of more than 200 people with diseases ranging from cancer, genetic vision loss, and amyloidosis. These are patients who undergo other treatments like chemotherapy, bone marrow transplant, and look to genetic therapy as their last attempt at a normal life.
However, not all volunteers made it through. Successful treatments are expensive, which limits the underprivileged and unwealthy. Furthermore, this process is still considered in the experimental phase and has not been approved, and not all treatments have been a success. The case of the ‘CRISPR babies’ demonstrates the dangers of germ-line testing. The ‘CRISPR babies’ had their CCR5 gene genetically modified by He Jiankui, to create HIV resistance. While CCR5 is a coding gene, this highlights the broader dangers and consequences of editing regulatory and non-coding sequences since the functions remain unclear.13

This led to the arrest of He Jiankui, and soon after, researchers discovered that the gene editing tools used were not perfect, and the “messy” edits caused unintended insertions, deletions, or rearrangements.14 These unintended edits often appear in non-coding regions that regulate gene expression or genome stability, which is a high concern since it can disrupt the regulatory networks and chromatin structure that keep the genome organized.5 Not only is this an issue for long-term reasons, but it also creates consensual issues for the human embryos. Despite the overall intention of preventing a detrimental disease such as HIV, the future of these individuals was violated, and their offspring are now at risk of genetic mutations. These examples reveal the tension between scientific progress and the ethical responsibility to protect human dignity.
Ethics and Human Responsibility
It is best to understand the overall picture of genomics and the ethics behind it. While advancements in science are a part of human nature, we should also understand the moral aspect that comes along with it. If only the wealthy can afford treatments, equality is now questioned. Genomic editing is beneficial in developing technology and targeting severe illnesses. On the other hand, genome editing also creates issues for future generations and formulates compensatory phenotypes. Moreover, chromatin accessibility and epigenetic regulation add layers of uncertainty: accessibility patterns can shift with age, environment, or exposure to chemicals, meaning that even a well-intentioned edit might have different effects in future generations or populations.5 There are working treatments for various diseases that have been working and improving the lives of individuals. As ideal as specific gene therapy sounds, it is a risk to open these therapies to individuals without understanding the effects. Furthermore, genome editing for humanity appears to be ideal, but it comes with a cost because it is still not fully developed or approved as a treatment. However, there will be cases with no effective treatments or life-threatening; in which carefully regulated somatic gene editing, can be justified as a last resort. So, despite the progress of genomics being vital, it must be fully transparent and respect the human body, especially since editing could have irreversible consequences.
Social Justice and Catholic Intellectual Tradition (CIT)
Now, both sides of this coin seek the best for human well-being. One through innovation and technology, while the other through caution. Although we do see the advantages of using CRISPR therapies in cases like Gray’s, there are still cases like the “CRISPR babies” where it reveals the dangers of moving beyond what we know and understand. Much like films that portray robots designed to simplify human life then exceed human control, gene editing exposes the inherent risks of pursuing technological progress without a full grasp of its wider consequences. In Gray’s case, editing the genome for sickle cell only on somatic cells did not
change her genome, meaning that her offspring will not inherit these edits. On the other hand, the CRISPR babies had edits made to the germline cells, potentially affecting both coding and noncoding regions, and thus can be inherited by future generations.17
Beyond the laboratory, editing non-coding regions introduces issues with social justice and equity. As mentioned before in the CRISPR editing for Gray, some people did not follow through with treatments because of the cost. Only a few wealthy nations can afford the resources needed for safe intervention. Without the correct infrastructure, health disparities will only increase due to the lack of knowledge. The Catholic Intellectual Tradition (CIT) stresses that genomic knowledge serves all people, but there should still be policies ensuring safety and support for those seeking such therapy. CIT offers solutions rooted in moral responsibility by advocating for ethical review boards, access to treatment, and a commitment to uphold human dignity throughout scientific advancement.18 Further, ethical progress also depends on access to editing genomic regions that translate to a societal responsibility to share this moral thought.
Theological and Ethical Reflection
Concerns arise when the discovery of non-coding regions was found to be responsible for gene regulation. While not fully understood, it is important to preserve their function and complexity. Yang Chen’s study emphasizes that accessibility maps provide the “control panels” for gene regulation, and these regions integrate signals from histone marks, DNA methylation, transcription factors, and 3D genome folding.5 Altering them without mapping interplay risks disturbing entire cellular networks, not just a single gene. Scientific advancements test and sometimes diminish the ethical limits that were set for human dignity and equity. Humans are beyond their brains and societal status. The CIT emphasizes that the human body is not merely a tool for education but a sacred expression of life.18
These teachings show that scientific inquiry must also align with justice and the common good. In this circumstance, when editing a genomic region, one must weigh the cost of moral values for the uncertainty of editing non-coding DNA. CIT encourages reflection not only on the potential to reverse an unintended mutation but also on the deeper question of what else might be altered in the process. Especially in non-coding regions of DNA, the good deed must outweigh the potential harm; therefore, if the risks outweigh the benefits, it fails the CIT test. Ethical discernment is not about what can be done, but about preserving the fundamental respect owed to every person.
Lonergan’s Framework of Responsibility

The ethics behind editing non-coding DNA regions involve relationships beyond the lab. Bernard Lonergan’s call to be responsible covers the importance of researchers and society both taking accountability. Within Lonergan’s framework, the first step is being attentive, which in this scenario would be carefully examining these “silent” genomic regions. Secondly, being intelligent, in this case, means interpreting how these edits in enhancers or non-coding DNA regions cross gene networks.21 Recognizing that editing genes can disrupt unseen gene networks, shows great ethical discernment that come along with innovation.
These imperatives remind researchers that there must be transparency and honesty before approaching the unknown. Applying Lonergan’s idea of attentiveness to modern genomics would mean mapping chromatin accessibility with tools like ATAC-seq or single-cell assays before intervening. This is an ethical step forward that ensures scientists understand when and where a region is active before attempting an edit.5 While discoveries are exciting, Lonergan’s framework reminds us that attentiveness and responsibility must be a foundation for excitement to grow.
Deontology and the CRISPR babies
He Jiankui intended to prevent individuals from getting HIV, but in the end, he did more harm than good. One who follows the ethical theory of deontology could say that his intentions were inherently good. In deontological ethics, intentions hold greater moral weight than outcomes.23 While Jiankui may have intended to find a solution to prevent HIV transmission, it remains ethically questionable that he misled the parents into giving consent, especially when other established treatments for HIV already exist. About 630,000 individuals died from AIDSrelated illnesses as of 2024, but 40.8 million individuals are still living with this disease.24 While the numbers are large, was it morally right and ethically sound for Jiankui to edit the target gene and unintentionally edit other regions of DNA? Through this thinking, you can cause more damage to an individual, ultimately violating their rights for one’s personal ambition or goal.
Final Thoughts to Consider
The question of whether editing non-coding regions of DNA is ethically acceptable is still unknown. Creation of CRISPR and degron systems proves and shows the technological advancements, while leaving the ethical lines blurred. Cases like Victoria Gray and “CRISPR babies” represent successes and the dangers that come along with them. This reveals the vast potential for growth in the knowledge we have yet to attain.
Chromatin accessibility studies reinforce that non-coding DNA is not static, but rather responsive, environmental, and dynamic. Thus, meaning any permanent genomic edit could shift regulatory balance far beyond what we can currently predict.5 Due to the little understanding about gene editing, the challenge is not based on whether it is ethical to edit a gene but on whether we have the right to make that decision. Should we treat an incurable disease like sickle cell, but not treat HIV, since there are living patients on current treatment? That is where the threshold in question is debated, especially since knowledge is so vast in the field. Ultimately, the question extends beyond genome editing and its moral justification. The Catholic Intellectual Tradition (CIT) and Lonergan’s moral framework expands that scientific progress must rely on humility, justice and respect for human dignity. As research in non-coding regions of DNA continues, the balance of science and ethics will determine the future of innovation
- MedlinePlus. “What Is Noncoding DNA?” U.S. National Library of Medicine, January 19, 2021. https://medlineplus.gov/genetics/understanding/basics/noncodingdna/. ↵
- Sen, Shurjo K. “Non-Coding DNA.” Genome.gov, October 6, 2025. https://www.genome.gov/genetics-glossary/Non-CodingDNA#:~:text=Non%2Dcoding%20DNA%20corresponds%20to,DNA%20have%20no%20known%20function. ↵
- “The Encyclopedia of DNA Elements (ENCODE).” Genome.gov, accessed October 7, 2025. https://www.genome.gov/Funded-Programs-Projects/ENCODE-Project-ENCyclopedia-Of-DNA-Elements. ↵
- “The Encyclopedia of DNA Elements (ENCODE).” Genome.gov, accessed October 7, 2025. https://www.genome.gov/Funded-Programs-Projects/ENCODE-Project-ENCyclopedia-Of-DNA-Elements. ↵
- Chen, Yang, Rui Liang, Yong Li, et al. “Chromatin Accessibility: Biological Functions, Molecular Mechanisms and Therapeutic Application.” Signal Transduction and Targeted Therapy 9, no. 1 (2024): 340. https://doi.org/10.1038/s41392-024-02030-9. ↵
- Elizalde, Mary Jane, and Daniel A. Gorelick. “Mechanistic Toxicology in Light of Genetic Compensation.” Toxicological Sciences 197, no. 2 (2023): 115–20. https://doi.org/10.1093/toxsci/kfad113. ↵
- Chen, Yang, Rui Liang, Yong Li, et al. “Chromatin Accessibility: Biological Functions, Molecular Mechanisms and Therapeutic Application.” Signal Transduction and Targeted Therapy 9, no. 1 (2024): 340. https://doi.org/10.1038/s41392-024-02030-9. ↵
- Fliesler, Nancy. “A Potential Danger of CRISPR Gene Editing—And Why Base Editing May
Be Safer.” Answers from Children’s Hospital, July 14, 2022. https://answers.childrenshospital.org/crispr-gene-editing/. ↵ - “CRISPR.” Accessed October 7, 2025. https://www.genome.gov/genetics-glossary/CRISPR. ↵
- Adhikari, Bikash, Ashwin Narain, and Elmar Wolf. “Generation of Auxin Inducible Degron (AID) Knock-in Cell Lines for Targeted Protein Degradation in Mammalian Cells.” STAR Protocols 2, no. 4 (2021): 100949. https://doi.org/10.1016/j.xpro.2021.100949. ↵
- Chen, Yang, Rui Liang, Yong Li, et al. “Chromatin Accessibility: Biological Functions, Molecular Mechanisms and Therapeutic Application.” Signal Transduction and Targeted Therapy 9, no. 1 (2024): 340. https://doi.org/10.1038/s41392-024-02030-9. ↵
- Page, Jessica Hamzelou. “More than 200 People Have Been Treated with Experimental CRISPR Therapies.” MIT Technology Review, accessed October 7, 2025.
https://www.technologyreview.com/2023/03/10/1069619/more-than-200-people-treated-with-experimental-crispr-therapies/. ↵ - Greely, Henry T. “CRISPR’d Babies: Human Germline Genome Editing in the ‘He Jiankui Affair’.” Journal of Law and the Biosciences 6, no. 1 (2019): 111–83. https://doi.org/10.1093/jlb/lsz010. ↵
- “Chinese Scientist Who Produced Genetically Altered Babies Sentenced to 3 Years in Jail.” Science, accessed October 7, 2025. https://www.science.org/content/article/chinese-scientist-who-produced-genetically-altered-babies-sentenced-3-years-jail. ↵
- Chen, Yang, Rui Liang, Yong Li, et al. “Chromatin Accessibility: Biological Functions, Molecular Mechanisms and Therapeutic Application.” Signal Transduction and Targeted Therapy 9, no. 1 (2024): 340. https://doi.org/10.1038/s41392-024-02030-9. ↵
- Chen, Yang, Rui Liang, Yong Li, et al. “Chromatin Accessibility: Biological Functions, Molecular Mechanisms and Therapeutic Application.” Signal Transduction and Targeted Therapy 9, no. 1 (2024): 340. https://doi.org/10.1038/s41392-024-02030-9. ↵
- Wiley, Lindsay, Mattison Cheek, Emily LaFar, et al. “The Ethics of Human Embryo Editing via CRISPR-Cas9 Technology: A Systematic Review of Ethical Arguments, Reasons, and Concerns.” Hec Forum 37, no. 2 (2025): 267–303. https://doi.org/10.1007/s10730-024-09538-1. ↵
- Ayre, Father Harrison. “Theological Anthropology 101: How Do We See the Body?” Simply Catholic, February 4, 2024. https://www.simplycatholic.com/theological-anthropology-101-how-do-we-see-the-body/. ↵
- Chen, Yang, Rui Liang, Yong Li, et al. “Chromatin Accessibility: Biological Functions, Molecular Mechanisms and Therapeutic Application.” Signal Transduction and Targeted Therapy 9, no. 1 (2024): 340. https://doi.org/10.1038/s41392-024-02030-9. ↵
- Ayre, Father Harrison. “Theological Anthropology 101: How Do We See the Body?” Simply Catholic, February 4, 2024. https://www.simplycatholic.com/theological-anthropology-101-how-do-we-see-the-body/. ↵
- Black, Taylor. “The Application of Bernard Lonergan’s Cognitional Structure in Ethical AI.” Church Life Journal, August 15, 2025. https://churchlifejournal.nd.edu/articles/bernard-lonergans-cognitive-structure-for-ethical-ai/. ↵
- Chen, Yang, Rui Liang, Yong Li, et al. “Chromatin Accessibility: Biological Functions, Molecular Mechanisms and Therapeutic Application.” Signal Transduction and Targeted Therapy 9, no. 1 (2024): 340. https://doi.org/10.1038/s41392-024-02030-9. ↵
- Barrow, Jennifer M., and Paras B. Khandhar. “Deontology.” In StatPearls. StatPearls Publishing, 2025. http://www.ncbi.nlm.nih.gov/books/NBK459296/. ↵
- “Global HIV & AIDS Statistics—Fact Sheet.” UNAIDS, accessed October 7, 2025. https://www.unaids.org/en/resources/fact-sheet. ↵
- Chen, Yang, Rui Liang, Yong Li, et al. “Chromatin Accessibility: Biological Functions, Molecular Mechanisms and Therapeutic Application.” Signal Transduction and Targeted Therapy 9, no. 1 (2024): 340. https://doi.org/10.1038/s41392-024-02030-9. ↵


