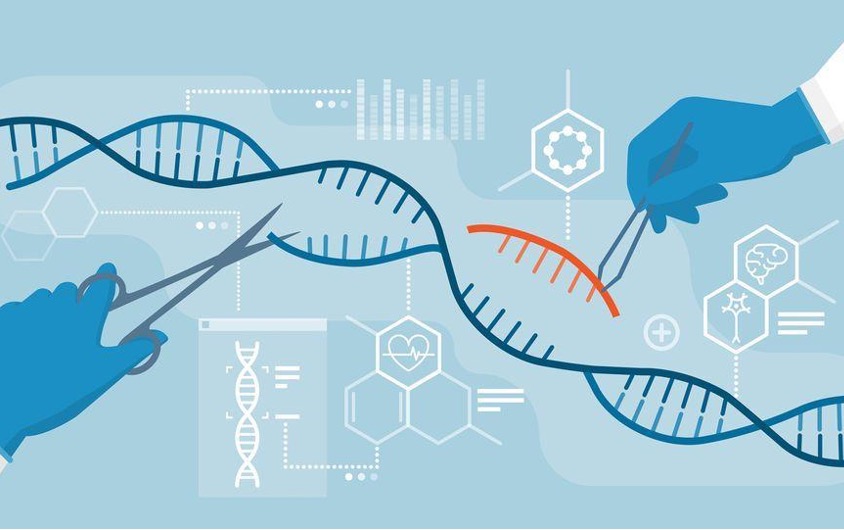
Genetic engineering is a powerful tool that can improve livelihood in humans, as well as efficiency in agriculture. This topic has been around for years, however the idea has resurfaced after Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) was discovered. In this context, CRISPR-Cas is used specifically. Meaning the CRISPR has an associated nuclease. CRISPR-Cas is guided to its target by a guide RNA, binds to the DNA, and cuts the section that needs to be edited. It originated in removing viruses from DNA to disable them. CRISPR-Cas has become important in rewriting code in organisms (Synthego 2024). Experiments have been done on both humans and crops, but there is hesitation with opening access to more people. The hesitation focuses on the idea that those with the finances to access this technology, may not use it for good and may take advantage. While genetic engineering can improve health and agriculture productivity, many believe it should be limited to disease prevention and essential medical treatment, with strict regulation to ensure human dignity, safety, and the common good.
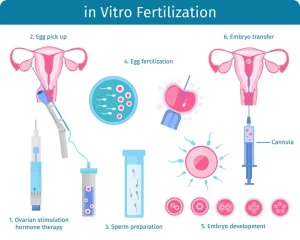
The main idea in genetic engineering on humans is to prevent illness and improve medicine. One of the earliest human experiments of genetic engineering happened in London through in vitro fertilization (IVF), which allows clinicians to screen embryos for harmful mutations.. IVF is a procedure of combining sperm and multiple eggs outside of the body in a lab. The woman is first given medicine to stimulate egg production. The eggs are then retrieved and placed with sperm. Once an embryo is produced, it is transferred to a culture to be observed. Then it is transferred to the woman’s womb to fertilize. (MedlinePlus 2022). All of the women in the father’s family passed away from breast cancer in their 20s. The trend stems from the BRCA-1 gene mutation in the ancestry. The gene codes for breast cancer type 1, correcting any errors in the helix so they function normally. If the gene is mutated, the risk of breast cancer increases significantly. There are thousands of ways the gene can be mutated, but it is rare to occur in the entire human population (Rubio 2025). The BRCA-1 gene mutation was deleted from the embryo before it was implanted into the mother’s womb through IVF. The procedure was successful, and the baby girl was born without the gene in January of 2009 (Scowcroft, 2009). Soon after her birth, there was public backlash regarding ethical concerns. The majority of the concerns focus on the risks of IVF. The procedure is time consuming, can be negative emotionally, and it is costly. It can take multiple procedures to get an IVF embryo result in a live birth, which can affect the mother negatively (MedLine Plus 2022). There is also concern with the need for IVF. Some do not agree that the procedure was necessary, since the daughter is not 100% cancer free. Cancer treatments and survival rates have been improving, however the family history shows the likely result of the daughter’s survival if IVF was not done (Scowcroft 2009). If the child developed cancer later in life, it is theorised to be a treatable cancer, as that is most common in people without the BRCA-1 gene. However, this is not confirmed. Some discussed that there are other biological effects that could come from deleting this gene. There are no confirmed findings of how it would affect an individual’s phenotypes or how it could react with different environments. Therefore, this idea has not been a recommended choice to parents for severe cases, such as this one.
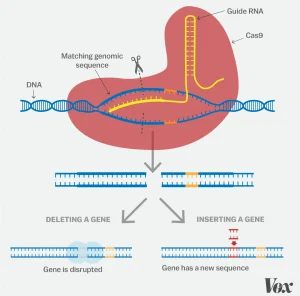
(Courtesy of Vox)
Another successful, but controversial, human experiment occurred in China. In 2014, there was research published using CRISPR-Cas9 on monkey embryos. They were able to achieve precise gene targeting in the one-cell-stage embryos (Niu, et .al, 2014). This research was significant, as monkeys and humans share a high percent of DNA. A couple years later in 2018, the first human experiment was performed. He Jiankui is a biophysicist who claimed to successfully delete the CCR5 gene, which can induce the HIV virus when present in twin embryos. CCR5 is a G protein-coupled receptor expressed in the immune system. HIV contains a lipid bilayer, which attaches to CCR5 to initiate infection (Barmania, 2013). The father of the twins was a carrier for HIV, so they decided to test if the gene could be removed to prevent the infection in the children. When news of the experiment was released to the public, it prompted a lot of backlash. “Ethical Guide Principles for Research on Embryonic Stem Cells” was implemented in China in 2003 which specifically bans in vitro research after the 14th day of existence (Raposo, 2019). It appears that Jiankui went against China’s guide to doing this research, and even though it was successful, it was risky. In CRISPR-Cas, any effects of the changes can not be controlled. Meaning, the changes may be beneficial, but the outcomes are not completely known. Many scientists believe the research should be limited to nonviable embryos, as they are impossible to result in a live birth (NHGRI, 2017). Given the risks, a lot of institutions, such as the National Institutes of Health, do not provide funding for this research. The lack of funding narrows the access to research to investigate the risks and remove them if possible.
Other debates revolve around the safety and unknown aspects about genetic engineering. The technology is advanced, however there is still a chance of off-target effects. This is when the editing happens in the wrong place, resulting in undesired or unknown effects. The procedure is tedious, and risking health is far more important than the possible benefits (NHGRI, 2017). There are also risks for future generations. Since the technology permanently alters the human genome of an individual, the offspring will likely carry the new material. There are no confirmed findings to determine how the editing will affect the gene pool or the population down the road. The main idea is to improve hereditary disease, however it can not be determined at this point in time with the lack of knowledge of long term effects. (Rubeis et al., 2018)
(Courtesy of LabXChange)
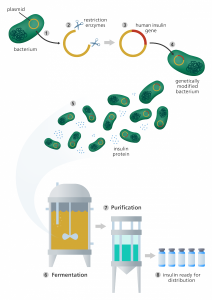
Instead of editing the human genome, some researchers have been engineering bacteria to turn into medicine. The first approved medicine in this aspect is insulin. The insulin prescribed is a genetically modified organism (GMO) medicine to replicate the insulin humans make in the pancreas. Insulin is a protein that helps break down genetic materials and turn them into energy needed for bodily functions (Assem, 2022). Besides the health benefits, there are also economic benefits. The genetically modified insulin can be produced in mass amounts, reducing the cost compared to other manufacturing processes. It also reduces the risk of adverse reactions (BBC, 2025). Adverse reactions are any side effects that occur outside of the intended purpose of taking a drug. Although there are still some public concerns about GMOs, insulin has repeatedly been proven safe to use.
The primary use of GMOs is in agriculture. GMOs can be created naturally through breeding and evolution or genetic engineering. Scientists can copy a desired gene in one organism, copy it, and insert it into the organisms they want to alter. GMOs are another example of using CRISPR (FDA 2024). Agriculture is the production and transportation of crops without disrupting the environment. Researchers have been testing which genes can be silenced or enhanced to improve this system (Aziz et al., 2022). One of the first digestible GM plants was tomatoes. The crops were modified to disrupt the production of the polygalacturonase enzyme, which is responsible for rotting. Some of the first GMOs to be FDA approved were cotton, corn, and potatoes (Aziz et al., 2022). Many farmers use plants with an enhanced Bt gene to resist pesticides and harmful insects. This gene produces a toxic protein for natural resistance (Raman, 2024). Removal of genes also helps the survival of the crops. The environment is improved and the farmers save money on material by reducing the amount of care needed to keep the crops alive. This includes less water, less pest resistant chemicals, and other chemicals needed for survival. If the crops themselves have a pest resistant gene, there is a significantly reduced risk of harmful chemicals getting into the products that are consumed.
After the crops are fully developed, they still have benefits afterwards. Researchers have discovered some can be modified to add or increase the amount of nutrients in the crops. This allows for people to consume healthier food, which may help with malnutrition and disease. An example of this is Golden rice. Beta carotene was added to help with Vitamin A deficiency. It also has been credited with promoting health and decreasing child morality in some countries (NCB, 2023). Many crops have mycotoxins that are extremely harmful to those who consume them. Connecting the pest-resistant gene to the fact that crops are used for consumption, the GMOs have reduced the risk of cancer and other illnesses (Smyth, 2019). If these types of products were more widely available, health benefits would likely improve significantly.
There is an important example that occurred in the early 1990s. Papayas are rich in demand, especially in Hawaii. Papaya ring spot virus became an extreme issue in this time, resulting in a quick decline in papaya productivity. This virus damaged the leaves, size, and quality of these fruits (Bawa, 2012). Without this engineering in the papayas, it is likely there would not be crops being produced today.
On the other hand, GMOs still have ethical concerns with humans and the environment. There are some editing procedures that give GMOs antibiotic resistant genes to survive. However, there is concern with how this will affect humans once the GMOs are consumed. The antibiotic resistant gene may be transferred to the person. If the person has or develops a disease, the new gene may interfere or counteract the effect of a remedy. An important aspect of the research to acknowledge, is that it is all short-term experiments. This means there are no known long-term effects to the human population (Abdul 2022). There is also concern for allergy reactions from the new products, since these changes do not occur in nature. To address this concern, there is some testing in vitro to determine if a GMO is safe for consumption (Bawa, 2012). However, this is not a widely available option, and there has not been too much testing previously. Therefore, the public has not concisely decided if the allergies are completely out of the question or not. The main issue with these concerns, is that they are hypothetical perspectives that have not been proven scientifically.
The ethical debate around genetic engineering can be further understood using the Lonergan’s GEM framework. Society does see the suffering caused by inherited diseases and the hope technologies offer through the experience of others or themselves. In understanding, we see the germline editing affects future generations that can not consent to these procedures. Society must weigh the reduction in suffering to the risks of social inequalities in which only the wealthy benefit. The decision requires policies that protect human dignity and the common good. Additionally, community discussion and ethical oversight are necessary to ensure these decisions reflect shared values rather than only scientific capability. This allows the balance of science and moral responsibility to collide and improve society as a whole. Genetic engineering can offer significant benefits in our society from agriculture to human health. However, the regulations are important, so the technology is not abused or taken advantage of.
Overall, genetic engineering is a highly debated topic with a lot of research to support both sides. It has the potential to reduce suffering, treat disease, and improve food security. However, it carries risks that affect future generations and the economic systems. Therefore, the use of genetic engineering should be heavily regulated to prioritize human safety and the common good, especially long-term. It should be limited to medical necessity and agricultural sustainability. Continued research, and public engagement is essential to develop policies that guide how these technologies can be used responsibly.
Citations:
Abdul Aziz, M., Brini, F., Rouached, H., & Masmoudi, K. (2022b). Genetically engineered crops for sustainably enhanced food production systems. Frontiers in Plant Science, 13(1), 1–24. https://doi.org/10.3389/fpls.2022.1027828
Assem, S. (2022, June 29). Genetic Engineering in Humans: 10 Pros and Cons – praxilabs. Praxilabs. https://praxilabs.com/en/blog/2022/06/29/genetic-engineering-in-humans-2/
Barmania, F., & Pepper, M. S. (2013). C-C Chemokine Receptor Type Five (CCR5): an Emerging Target for the Control of HIV Infection. Applied & Translational Genomics, 2, 3–16. https://doi.org/10.1016/j.atg.2013.05.004
Bawa, A. S., & Anilakumar, K. R. (2012). Genetically Modified foods: safety, Risks and Public Concerns—a Review. Journal of Food Science and Technology, 50(6), 1035–1046. National Library of Medicine. https://doi.org/10.1007/s13197-012-0899-1
BBC Bitesize. (2025). Genetic Modification – Uses in Medicine – Monitoring and Maintaining Health – Non-communicable – OCR Gateway – GCSE Biology (Single Science) Revision – OCR Gateway. BBC Bitesize. https://www.bbc.co.uk/bitesize/guides/zgfm3k7/revision/9
FDA. (2024). Science and history of gmos and other food modification processes. U.S. Food and Drug Administration, 1. https://www.fda.gov/food/agricultural-biotechnology/science-and-history-gmos-and-other-food-modification-processes
Ma, Y., Zhang, L., & Qin, C. (2019). The First Genetically Gene‐edited babies: It’s “Irresponsible and Too Early.” Animal Models and Experimental Medicine, 2(1), 1–4. https://doi.org/10.1002/ame2.12052
MedLine Plus. (2022, October 1). In vitro fertilization (IVF): MedlinePlus medical encyclopedia. Medlineplus.gov. https://medlineplus.gov/ency/article/007279.htm
National Human Genome Research Institute. (2017, August 3). What Are the Ethical Concerns of Genome Editing? National Human Genome Research Institute; National Institutes of Health. https://www.genome.gov/about-genomics/policy-issues/Genome-Editing/ethical-concerns
Nebraska Corn Board. (2023, December 5). Five Surprising Benefits of GMOs. Nebraska Corn Board. https://nebraskacorn.gov/cornstalk/food/five-surprising-benefits-of-gmos/
Raman, R. (2024, January 9). GMOs: Pros and Cons, Backed by Evidence. Healthline. https://www.healthline.com/nutrition/gmo-pros-and-cons
Raposo, V. L. (2019). The First Chinese Edited Babies: A Leap of Faith in Science. JBRA Assisted Reproduction, 23(3), 197–199. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6724388/
Rubeis, G., & Steger, F. (2018). Risks and Benefits of Human Germline Genome editing: an Ethical Analysis. Asian Bioethics Review, 10(2), 133–141. https://doi.org/10.1007/s41649-018-0056-x
Rubio, M. (2025, February 16). What is BRCA1? About the BRCA1 mutation and more | BCRF. Breast Cancer Research Foundation. https://www.bcrf.org/about-breast-cancer/brca1/
Scowcroft, H. (2009, January 30). “BRCA1-free” Birth Isn’t a “slippery Slope to Designer babies.” Cancer Research UK – Cancer News. https://news.cancerresearchuk.org/2009/01/30/brca1-free-birth-isnt-a-slippery-slope-to-designer-babies/
Smyth, S. J. (2019). The Human Health Benefits from GM Crops. Plant Biotechnology Journal, 18(4). National Library of Medicine. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7061863/
Synthego. (2024). The ultimate guide to CRISPR: Mechanism, applications, methods & more. Synthego. https://www.synthego.com/learn/crispr