In the past few decades, the swift advancements that have been made within genetic research have played a noteworthy role in the knowledge of disease, medicine, biology, and evolution that researchers have been able to possess over time. However, another aspect of genetic research that is noteworthy is the different communities and groups that said research is conducted in, specifically the marginalized communities that genetic research is conducted in. As we explore further, it is apparent that there is an array of ethical concerns and discussions that have been presented against or in defense of genetic research in marginalized communities. While some may seem to clash or contradict, others may appear to go hand in hand.
As mentioned previously, genetic research has resulted in a great amount of advancement in biomedical research specifically.
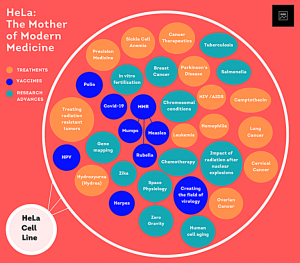
For instance, in recent years genetic research has led to a handful of serious diseases such as sickle cell disease and Duchenne muscular dystrophy having several disease models for biomedical study. (Zhou et al., 2022) These disease models ae However, when thinking of ethical concerns that apply to genetic research, there is the question of if conducting such research withing marginalized communities can lead to the exploitation of the individuals within those communities. One prime example that many use to explore this idea is the case of Henrietta Lacks. Lacks was a young African American mother of five who was diagnosed with cervical cancer in the 1950’s. While receiving care at Johns Hopkins Hospital, Lacks’
accumulated tissue cells from all of her tests and treatments were given to researchers without her consent. These researchers quickly discovered that her cells had the capability of surviving outside of the human body and despite Henrietta passing soon after, her cells are still used till this day in several types of scientific research, (now famously named HeLa cells) while Henriettas family has never received any form of financial compensation or benefits, resulting in a handful of her family members to still live in poverty.(Beskow, 2016) Throughout Henrietta’s case, there is a common theme that can be further observed in the examination of genetic research within marginalized communities; a lack of consent.
When pondering the ethics of genetic research within disadvantaged communities, a lack of consent from those participating in the studies is a common trend that still impacts the field of genomics to this day. In fact, in a recent survey, people were asked about their trust level within the sharing of genomic data, the individuals were categorized into “high trust” and “low trust” groups, with 43% of the participants being categorized as “low trust”. It is noteworthy that while there is a slight majority of individuals in the “high trust” group, a majority of the individuals who were high trust were males who were under 50 years old, held religious beliefs and already had a personal experience of genetics. (Milne et al., 2019) This piece of
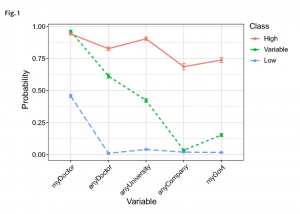
information slightly suggests that those who you would not typically categorize as marginalized or disadvantaged (young, male, prior or at least basic knowledge of genomics) seem to have an easier time trusting genetic research because they are not weary of their information, cells, data, etc., being used without their consent, while those who could be considered apart of disadvantaged communities (elderly, women, those who do not have access to the same education) tend to have a lack of trust in genetic research out of concern that they will not be given the option to consent. While this disparity of trust within individuals and genetic research makes it clear that the conversation about ethics in genetic research is valid, it is also crucial to discover the benefits of genomic research in marginalized communities.
As stated before, genetic research has led to several advancements within biomedicine, however, there are also advancements that have come specifically from genetic research done in disadvantaged communities. A great example would be the Alabama Genomic Health Initiative (AGHI). The AGHI is a state-government-funded initiative created in 2017 that performs genomic testing on communities within the state of Alabama. Because it is a service that is free to all Alabama residents, it specifically benefits the disadvantaged and has resulted in some significant discoveries and advancements in the few years it has been around. For instance, in a study conducted by those at the AGHI, two groups were selected for genetic testing, one being the ”rare disease group” and the other being the “population screening group”.
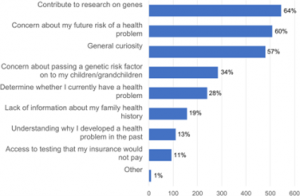
The researchers found that 19.9 percent of the rare disease group possessed a genetic variant that could contribute to disease and 1.5 percent of the population screening group had positive genotype results for several diseases such as cancer, hypercholesterolemia and cardiomyopathy. (Genetic Discrimination and Misuse of Genetic Information, 2022). This initiative resulted in the betterment of those who may be disadvantaged in a community by improving the speed at which a prominent illness or disease is detected within that community and further creates a sense of fairness in genetic healthcare between the advantaged and the disadvantaged, which presents the concept that genetic research must be conducted in all communities to avoid inequality in genomic healthcare.
Interestingly, in results from 2019 genome wide association studies, a 78% majority of participants were of European ancestry, while only 10% were of Asian descent, 2% of African descent, and only 1% of Hispanic descent. (Tawfik et al., 2023) The lack of equal genetic study in this case displays how not performing genomic testing withing marginalized communities is another aspect of ethical concerns within genetic research. While equity is what most would consider a non-negotiable within healthcare (in this case genetic healthcare), there is interesting discrepancy here that may tell a story on the ethics of genetic healthcare. Genetic research can allow healthcare providers the ability to detect diseases and illnesses early and give patients a fighting chance, and one could say that in order for everyone to have that fighting chance, genetic research has to be conducted within disadvantaged or marginalized communities. One well known example of a disease that can be caught early is cancer, many hereditary cancers can be detected early in individuals via genetic testing and treatment can begin for them right away to give them a better chance at remission.
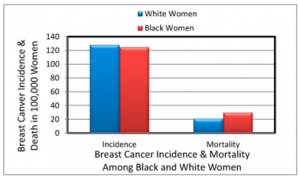
This is also a point that makes another case for the need to provide genetic healthcare to all including those is disadvantaged communities because according to author Jen Miller, those with cancer who are apart of marginalized groups have higher mortality rates than those of other groups. For example, black women have higher death rates for cervical and breast cancer despite having almost equal rates of the disease as other races of women, while black men are more likely to die from prostate cancer compared to men of other races. (Miller, 2024)
With that knowledge it is worth noting the possibility that ensuring genetic testing easily accessible for every community would result in everyone benefiting from diseases being detected early, therefore bridging the gap in disease mortality rates between different communities, as the difference between incidence and mortality rates is a result that must be considered when discussing the ethics of genetic testing and research.
Genetic research allows for so many advancements within several aspects of the healthcare and biomedical research that is available in our society. While the advancements, breakthroughs, and innovations must be acknowledged, one must also consider that there will always be the ethical concerns that surround genetic research when it is conducted in the communities of those who are already at a disadvantage in society. What can be said for every concern within this topic is that there is a possible solution, whether it be those in genetic healthcare having a change in ethical policy, or the creation of government-funded programs providing free genetic healthcare and testing just like the AGHI that was presented earlier. Another aspect to consider is the “grey area” on the topic. To further explain, it is worth noting that while there is a concern of those in disadvantaged communities not having the same amount of access to genetic healthcare as others, the other concern of those individuals being exploited or taken advantage of after participating in genetic testing in a way could appear to be contradictory. If marginalized groups do not have the same access to genetic research and testing as others, how are they being exploited due to genetic research being conducted in their communities? It is also notable that what communities may be consider marginalized or disadvantaged to people of the U.S, may not be considered the same in another country. And even within the U.S, the term “marginalized” or “disadvantaged” can be subjective to some, therefore the opinions on the ethics of performing genetic research in marginalized or disadvantaged communities can be vastly different from person to person. Aside from location, ones opinion on what is considered marginalized or disadvantaged could also depend on culture, economics within an individual’s location, or even ones social status. What one considers a marginalization or disadvantage, another might consider a standard or a privilege. With a subject as complex as this, there cannot be a simple two-sided argument when discussing it, this subject cannot be looked at as an argument, but rather a complex discussion that truly is just seeking what individuals think is morally right and ethical within our healthcare system. Genetic research is still developing and changing each and every day within our healthcare system, therefore ones observations and critiques on the ethics of it will constantly develop and change as well. In conclusion, the ethical conversations on genetic research within marginalized and disadvantaged communities is ever evolving as the field itself evolves. As the ethical concerns that are brought to the table today may not be the same ones presented later, and ones that may have been seen as resolved in the past could possibly come back around as the healthcare system continues to change in all sorts of ways. That being said, with all of these concerns and discussions around ethics in this field, it is imperative that those within genetic healthcare uphold the standard of treating each and every individual that is receiving genomic care with respect, privacy, and equity.
Citations
Beskow, L. M. (2016). Lessons from HeLa Cells: The Ethics and Policy of Biospecimens. Annual Review of Genomics and Human Genetics, 17, 395–417. https://doi.org/10.1146/annurev-genom-083115-022536
Genetic Discrimination and Misuse of Genetic Information: Areas of Possible Discrimination, Current Legislation, and Potential Limitations | Blogs | CDC. (2022, October 3). https://blogs.cdc.gov/genomics/2022/10/03/genetic-discrimination/
Miller, J. A. (2024, January 29). Changing the System: Marginalized Communities and Cancer. InventUM. https://news.med.miami.edu/cancer-and-marginalized-communities/
Milne, R., Morley, K. I., Howard, H., Niemiec, E., Nicol, D., Critchley, C., Prainsack, B., Vears, D., Smith, J., Steed, C., Bevan, P., Atutornu, J., Farley, L., Goodhand, P., Thorogood, A., Kleiderman, E., Middleton, A., & on behalf of the Participant Values Work Stream of the Global Alliance for Genomics and Health. (2019). Trust in genomic data sharing among members of the general public in the UK, USA, Canada and Australia. Human Genetics, 138(11), 1237–1246. https://doi.org/10.1007/s00439-019-02062-0
Tawfik, S. M., Elhosseiny, A. A., Galal, A. A., William, M. B., Qansuwa, E., Elbaz, R. M., & Salama, M. (2023). Health inequity in genomic personalized medicine in underrepresented populations: A look at the current evidence. Functional & Integrative Genomics, 23(1), 54. https://doi.org/10.1007/s10142-023-00979-4
Zhou, W., Yang, J., Zhang, Y., Hu, X., & Wang, W. (2022). Current landscape of gene-editing technology in biomedicine: Applications, advantages, challenges, and perspectives. MedComm, 3(3), e155. https://doi.org/10.1002/mco2.155



