According to the National Human Genome Research Institute, “around 350 million people on earth are living with rare disorders”.1 Many, or most, of these disorders are genetic disorders, meaning that an abnormality in a person’s DNA, or genome, has caused improper development in-utero.2 Many of these abnormalities are single nucleotide changes, insertions and deletions of DNA sequences, variation in chromosome structure, and repeats of various DNA sequences.3 These slight changes alter or may eliminate certain genes, resulting in dysfunctional, or lack of gene products. Stability of the genome is crucial in development; any alteration in the genome can result in diseases that range in severity. Some may result in slight intellectual disability like Down syndrome, in treatable but chronic conditions like cystic fibrosis, and some may result in early death such as Edwards syndrome.
Genetic conditions that result in early death, in-utero or post-partum, is an unfortunate reality that many parents have to face around the world. In many cases, parents may be knowledgeable about a genetic disorder their child possesses through genetic testing but have no way of helping them, coming face-to-face with the sad reality that they must prepare for their child’s death before they are born. However, what if there was a way to change this outcome? What if there was a way to change or correct the genome of developing fetuses to give them a chance of survival? The answer to these questions is the complex and controversial method of germline gene editing. Germline gene editing is a method where gene-editing tools are used to alter the genetic code of germ cells or embryo, allowing the resulting cells and their lineage to carry this change and produce normal, functional cells.4 This method can provide hope to prospective parents by putting the power to help their child back in their hands, although it is acknowledged that this ability may contribute to genome editing of healthy babies for purposes other than needed. In-utero gene editing should only be done in cases where there is no chance of fetal survival to ensure that use of this method is restricted to cases that warrant its use and does not lead to cosmetic editing.
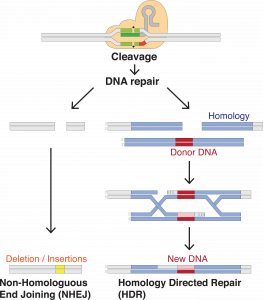
Gene editing is the modification of specific DNA sequences that code targeted genes. In the context of genetic disorders, modifications are made to repair any abnormalities by inserting or removing specific base pairs or sequences. These repairs are often facilitated by common enzymes or processes used in normal DNA replication. In many cases, a synthesized clone of a normal gene is introduced to the cell along with an enzyme that cleaves the DNA at a specific sequence. This allows for the synthesized DNA to replace the abnormal DNA, producing functional gene products.5
Alteration of the genome can be targeted during development in specialized somatic cells, or in germline cells or embryos. Somatic cells are cells within the body that are not involved in sexual reproduction; such as muscle cells, neurons, and skin cells. If altered, all daughter cells carry the genetic change, and it is passed through each generation. This alteration stays within the scope of the cell, typically in one tissue. Germline cells are cells within the body that are directly involved in sexual reproduction, such as sperm and eggs. The alteration of cells containing germ cells, such as embryos, is also included under this classification. As germline cells are not yet specialized to a specific tissue, the altered genetic information is carried by every cell throughout the body. Additionally, the altered genetic information is passed on to offspring.6
As gene editing is a relatively new method, use has been limited largely to animals. However, there have been many successful incidents in the use of animals. Although a different species, animal models can prove useful in predicting any effects that may occur to humans as many organ systems between both species are similar. In a study using mice, genetic modification of stem cells was proven to aid in the correction of multiple genetic mutations.7 In this study, non-specialized stem cells from an embryo were cultured and altered outside the body. The alteration of genetic information was facilitated by homologous recombination using an isolated, normal gene from a different organism. That is to say, a gene was introduced during meiosis, when condensed genetic information called chromosomes are lined up with other chromosomes containing the same genes, and segments are switched. This switch incorporates the normal gene into the cell’s genome and is effectively replicated for every cell division after. This study led to the development of more advanced methods suitable for use in larger non-rodent species.
However, the uneasiness of new methods pushes parents, and researchers, to use a more common or familiar technique. One popular method that is available to parents who are at risk of passing a genetic disease to their children is preimplantation genetic testing. This method is the biopsy of an early embryonic cell, such as a blastocyte, and sequencing its genome for any known variation that can cause genetic disorders.8 This is a favored method as it has been proven to accurately identify genetic abnormalities and gives parents the power to choose an embryo that has a greater chance of survival through in-vitro fertilization. However, this does not account for parents who did not know they carry a genetic abnormality, or for those whose children spontaneously screened positive for these disorders with no family history.
In cases where a baby is born with a genetic abnormality, somatic gene editing is the next step for parents in helping their child. Although a relatively new method, clinical trials have proven its efficacy and safety. Most recently, in February 2025, an infant underwent personalized somatic gene editing to fix an abnormality of a gene that affected their ability to break down ammonia in the liver, causing a toxic accumulation in the body. This abnormality, if left untreated, typically results in serious conditions such as damage to the brain and liver, coma, or even death. Fortunately, after receiving treatment, the infant has not seen any side effects typically associated with the disease, has decreased the number of medicines to treat ammonia accumulation, and has started a normal diet.9
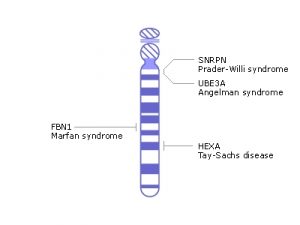
What about diseases that are not localized? Diseases such as Tay-Sachs disease which affect neurons in the brain and spinal cord, sickle cell anemia which affects all red blood cells in the body, or Cystic fibrosis which affect all mucus membranes that line multiple organs.10 Targeting all the cells in multiple regions of the body is much more complex and difficult than targeting cells in one organ. In cases where the disease affects the whole body, or a large part, germline editing can be observed to be more efficient. The treatment of a few cells during development turns into treatment of the whole body, avoiding progression of deadly or debilitating diseases completely. However, this is not an argument that germline gene editing is superior to somatic gene editing in any way, as it was previously mentioned that somatic gene editing is effective, it is simply a discussion to show that these methods cater to different situations. In a situation where a child would suffer from a full-body disease, the germline gene editing method would be more beneficial.
To rid a child of future suffering due to a genetic disease is a hope that parents hold dear, but what defines a genetic disease that constitutes germline editing? The boundary between necessary gene editing and unnecessary gene editing is a murky area where researchers and parents struggle to define. Diseases that are lifelong conditions such as Down syndrome or Turner syndrome can cause health problems but are generally manageable; people with these conditions live normal, long lives. With the ability to potentially fix the genetic abnormalities that cause these conditions, is there justification not to do so?
Like all new developing treatments, unknown side effects may occur while altering the genome. One popular, and major, worry is non-specific alteration of the genome or unintended consequences of the altered gene.11 As gene editing often involves the use of probes to guide the cleaving enzymes or synthesized DNA to its location, the possibility that incorrect locations are altered is plausible. This alteration could bring changes that result in harmful effects, further affecting the health of the child. Another possibility is the edited gene resulting in effects that do not affect the child until later in life. This worry is compounded when the offspring of a genetically modified individual is born. If an individual’s genome were altered and a mistake occurred, all children from this individual would carry this new genetic abnormality as well. However, all innovative technology and methods are improved as testing progresses, decreasing risk of side effects and indirect consequences.12 The termination of this method before further research can progress would mean losing the ability to save many lives in the future.
As germline editing is a new and unprecedented technology in healthcare, with only trials in small animals, in cases where a child is afflicted with mild disorders that do not affect their quality of life, the risk of this method is not justified. The ability of a child, or anyone, to live is prioritized in healthcare. The use of germline editing should not be risked for manageable disorders or non-treatment use. The goal of treating genetic disorders that only result in fatalities would reduce subjective use and ban the creation of personalized, designer babies.
Germline gene editing is a method that continues to be researched and debated around the world due to its direct, impactful effects on the lives of animals and possibly humans in the future. Although current methods exist in cases where the parents know of their child possibly inheriting a genetic disorder, germline gene editing aids parents of children in-utero. The insertion or deletion of specific genes and sequences during fetal development gives researchers the ability to give the gift of life in situations where the parents are powerless in helping their child. Acknowledging that this method may be used for subjective reasonings that are non-medical and may have side-effects that can give rise to more mutations in the genome; germline gene editing should be strictly regulated for use in fatal diseases to ensure this method is only used when warranted.
- National Human Genome Research Institute. (2018, April 13). Rare Genetic Diseases. Genome.gov. https://www.genome.gov/dna-day/15-ways/rare-genetic-diseases. ↵
- Mattar, C. N. Z., Chew, W. L., & Lai, P. S. (2024). Embryo and fetal gene editing: Technical challenges and progress toward clinical applications. Molecular therapy. Methods & clinical development, 32(2), 101229. https://doi.org/10.1016/j.omtm.2024.101229. ↵
- Jackson, M., Marks, L., May, G. H. W., & Wilson, J. B. (2018). The genetic basis of disease. Essays in biochemistry, 62(5), 643–723. https://doi.org/10.1042/EBC20170053. ↵
- Ormond, K. E., Mortlock, D. P., Scholes, D. T., Bombard, Y., Brody, L. C., Faucett, W. A., Garrison, N. A., Hercher, L., Isasi, R., Middleton, A., Musunuru, K., Shriner, D., Virani, A., & Young, C. E. (2017). Human Germline Genome Editing. American journal of human genetics, 101(2), 167–176. https://doi.org/10.1016/j.ajhg.2017.06.012. ↵
- Khalil, A. M. (2020). The genome editing revolution. Journal of genetic engineering and biotechnology, 18(1), 68. ↵
- Belete, T. M. (2021). The Current Status of Gene Therapy for the Treatment of Cancer. Biologics: Targets and Therapy, 15, 67–77. https://doi.org/10.2147/BTT.S302095. ↵
- Tang, L., González, R., & Dobrinski, I. (2015). Germline modification of domestic animals. Animal Reproduction, 12(1), 93–104. ↵
- Greco, E., Litwicka, K., Minasi, M. G., Cursio, E., Greco, P. F., & Barillari, P. (2020). Preimplantation Genetic Testing: Where We Are Today. International Journal of Molecular Sciences, 21(12), 4381. https://doi.org/10.3390/ijms21124381. ↵
- Musunuru et al, “Patient-Specific In Vivo Gene Editing to Treat a Rare Genetic Disease.” N Engl J Med. Online May 15, 2025. DOI: 10.1056/NEJMoa2504747. ↵
- Amato, P., Mikhalchenko, A., & Mitalipov, S. (2025). The case for germline gene correction: state of the science. Fertility and sterility. ↵
- Rubeis, G., & Steger, F. (2018). Risks and benefits of human germline genome editing: An ethical analysis. Asian bioethics review, 10(2), 133–141. https://doi.org/10.1007/s41649-018-0056-x. ↵
- Savulescu, J., Pugh, J., Douglas, T., & Gyngell, C. (2015). The moral imperative to continue gene editing research on human embryos. Protein & cell, 6(7), 476–479. https://doi.org/10.1007/s13238-015-0184-y. ↵