The Double-Edged Helix: Ethical Complexities of Gene Therapy in Modern Medicine
In recent decades, Gene therapy has emerged as a transformative approach in medicine, yet it has ethically complex frontiers in modern medicine. Once dismissed as an unrealistic dream, the ability to alter genetic material to treat or even cure hereditary diseases has rapidly evolved from an experimental concept to a clinical reality. Groundbreaking studies in gene therapy capture the excitement and uncertainty it can bring. For example, with the development of advanced genome tools editing tools such as CRISPR-Cas9 and approved treatments for disorders like sickle cell disease, gene therapy has crossed a threshold into widespread clinical practice. While the scientific advancements are promising, the ethical implications surrounding gene therapy are complex and multifaceted. Despite its promise, gene therapy raises complex ethical questions and issues that extend beyond the laboratory, particularly those associated with gene editing. The high costs associated with these treatments, the number of people who have access to specific modifications that could prolong someone’s life span or provide a better way of life, in some cases, gene therapy has detrimental effects on their health, their uncertain outcomes, and the potential to deepen existing healthcare disparities, making ethical evaluation essential. Gene therapy offers numerous benefits, including the potential to cure diseases previously considered incurable. However, there are ethical concerns at the same time, which make ethical evaluation essential. Gene therapy has vast amounts of potential, but should be handled with responsibility, fairness and humanity guiding its use.
Human gene therapy is defined as the transfer of nucleic acid in a patients somatic cells by either correcting genetic defects or by overexpressing proteins that are therapeutically beneficial (The Future of Human Gene Therapy, 2001). It aims to correct genetic defects or to express therapeutically useful gene products. One of the most widely used methods in gene therapy is recombinant DNA technology, in which a healthy gene is inserted into a carrier known as a vector. These vectors can be plasmid-based, nanostructured, or viral. Viral vectors are the most commonly used because of their high efficiency in entering cells and delivering genetic material. There are two categories: germline gene therapy and somatic cell gene therapy (Li et al., 2023) .Germline gene therapy is when reproductive cells, such as sperm or eggs, are genetically modified through the functional gene that integrates into the genome. These alterations are heritable and can be passed down through the generations, which provides a long-term solution to genetic and hereditary disorders. In contrast, Somatic cell gene therapy involves transferring healthy genes into a patient’s non-reproductive cells, which only affects the treated individual and is not inherited. Gene therapy involves DNA recombinant, where a functional “normal” gene is introduced into a patient’s genome to replace or compensate for a defective gene responsible for a specific disease. This therapeutic strategy aims to correct the underlying genetic cause rather than merely treat the symptoms. However, one technical challenge gene therapy faces are delivering the gene into the target stem cells, which are often difficult to transfect. To overcome this, researchers use molecular carriers known as vectors. Vectors transport and release the healthy gene into the target cell(The Future of Human Gene Therapy, 2001). There are multiple benefits of gene therapy such as, correcting the root cause of disease, instead of treating the symptoms, gene therapy aims to fix the faulty gene directly causing the problem and is only necessary to get done once providing a long term, potentially permanent benefit for the patient’s genetic disorder. Even though there are multiple benefits got gene therapy there are concerns people should beware of, including: risk of immune response, technical delivery challenges, as previously explained the viral vectors are often used to deliver genes into the cells can trigger a strong immune response which can affect the patient’s body, the range can be mild inflammation or organ failure. The viral vectors can also target the wrong cell for instance , it can go inside a normal healthy cell rather than the targeted cells which can lead to detrimental effects on the human body.
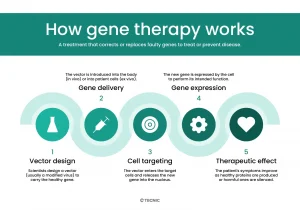
(courtesy of Tecnic)
There are 1800 genes associated with rare diseases that have been identified; their expression patterns and functions have been unraveled. In some cases, gene therapy is possible depending on the disease’s severity, feasibility, and treatment alternatives. Three things need to be established: whether the mutation leads to a gain or loss in function, its impact on cell survival or development, and the gene’s tissue specificity. There are four primary strategies for gene therapy, including the addition of a normal mutation that compensates for the absence or inactivity leading to a disease. This follows DNA recombinant, which involves inserting a functional version of the gene into a vector. The second strategy involves modifying mRNA to avoid the effects of mutation. This is promising when the affected exon is not needed for gene function. By altering or skipping the mutated gene during mRNA processing, the cell can still produce a functional protein, which can reduce disease severity. The third strategy focuses on inhibiting the expression of a mutated gene to prevent the production of toxic proteins. This is achieved through siRNA, which targets and degrades mutant mRNA before translation. The fourth is direct gene repair, which corrects the gene itself. This method relies on engineered chimeric proteins composed of a DNA-binding domain linked to a nuclease, such as FokI, that induces site-specific double-strand breaks in DNA. Once the break is created, a template containing the wild-type sequence is introduced, allowing the cell’s own homologous recombination machinery to repair the defect with the correct genetic information(Fischer & Cavazzana-Calvo, 2008).
Therapeutic gene transfer holds the promise of providing lasting therapies and even cures for diseases that were previously untreatable or for which only temporary or suboptimal treatments were available. Many people have benefited from gene therapy, such as modifying defective genes that cause genetic disorders such as sickle cell anemia or cystic fibrosis. Gene therapy can also introduce genes that enhance the body’s ability to combat diseases, viruses, or infections, such as HIV and cancer. Cancer cells can become more sensitive to radiation therapy or chemotherapy. Clinical gene therapy was marked by some impressive, albeit rare, successes and several setbacks. However, effective and long-lasting treatments are now being reported from gene therapy trials at an increasing pace. Positive outcomes have been documented for a wide range of genetic diseases (including hematological, immunological, ocular, and neurodegenerative and metabolic disorders) and several types of cancer. Examples include restoration of vision in blind patients, eradication of blood cancers for which all other treatments had failed, correction of hemoglobinopathies and coagulation factor deficiencies, and restoration of the immune system in children born with primary immune deficiency(Kumar et al., n.d.). Gene therapy has improved many people’s lives. One of the examples they included about improved vision in blind people involved injecting healthy genes into the patients’ retinas to repair the defective genes. By doing this, they have improved sensitivity to light. This can alter someone’s life for the better. Another example they mentioned was the eradication of blood cancers; gene therapies can modify the immune system cells and correct the defective cells as well.

(Courtesy of Prezi)
While gene therapy has many benefits, it raises a range of ethical concerns. One major issue that arises is off-target effects, which could cause insertional mutagenesis or an unintended gene mutation, potentially leading to tumor development, especially when using viral vectors. This can occur when it targets other spots on the genome rather than the intended target sites. A reason this could have occurred is by using a gene editing tool that binds to and cuts similar-looking DNA sequences. These effects can lead to genomic instability, resulting in harmful mutations or large deletions that are incredibly harmful to the genome and have long-lasting effects. In germline editing, the perseverance of gene editing vectors can develop into genetic mosaicism, where different edit can be carried in different cells, which results in developmental disorders, abnormalities, or hinder embryo development (Ansah, 2022). Many women, especially, do not know the side effects gene therapy can have on the human body. This could hinder someone’s ability to carry a baby to term, or the baby can have detrimental effects that could affect them for the rest of their life. This raises the concern of the moral responsibility of the hospital doctor to inform the patients of the harmful effects germline editing could have not only on the patients’ health but as well as the baby. This leads to another issue, which is informed consent. Germline editing raises questions about the rights of future generations who cannot give consent to those modifications made before their conception. This introduces debate over parental authority, professional responsibility, and the ethical limits on decision-making for embryos. So, this raises many problems and brings up the controversy of gene therapy. As stated, previous gene therapy could have effects on the embryo, which raises concerns for most people. The embryo has no say in what is being edited to improve its being of life, or it can cause harm. This is the problem for most; some patients are unaware of the consequences of gene therapy, but for the parents, the pros outweigh the cons. Whether or not the parental figure has authority over the doctor, ultimately, the decision is left to the patient. The doctor will provide information that will help the patient make that decision. This raises concerns about transparency between the researcher and the patient. For example, ‘’in SCD patients and caregivers were unwilling to join clinical trials, because historical events and prior family experiences led them to fear that researchers would be untruthful about or would conceal useful information from (minority) patients’’ (van Hooff et al., 2025). People have many concerns about researchers or doctors withholding information from the patient. Usually, the majority of the population that is being affected by most of these issues is the minority and of lower economic status. The mistrust between doctors or researchers and patients has been an ethical problem for many generations. Doctors withhold certain information that could cause problems later for the patients, such as impacting the patient’s safety or the emotional stakes it could have. This could cause serious consequences for the patients’ health and lead to legal issues for the doctor if they fail to disclose information to the patient. Another major ethical concern is gene therapy pricing and access, ‘’due to commercialization and the absence of strong competition, the process of calculating a price for these therapies is not disclosed, leaving much room for profit to the pharmaceutical companies’’(Gonçalves & Paiva, 2017). This lack of transparency in pricing raises questions about fairness, accountability, and public trust. When companies withhold cost information, patients and healthcare systems cannot assess whether prices are justified. High costs and limited accessibility affect patients from lower economic status, as well as marginalized populations. Lifesaving treatments should not be available to those who can afford them. Healthcare that can improve people’s way of life should be accessible to everyone. They should not be only for the select few. Healthcare systems have an ethical responsibility to ensure that medical breakthroughs benefit the entire population, not just those with wealth or privilege. Access to treatments should never be determined by income, location, or insurance status. For gene therapy to be recognized as an ethical form of care, fairness in accessibility must be a guiding priority. Providing fair, and access to these treatments also upholds the internationally recognized right to healthcare for everyone. The question is no longer whether gene therapy works, but instead for whom, at what cost, and under what moral circumstances. The catholic intellectual tradition emphasizes the integration of faith, reason and moral reflection. In gene therapy catholic intellectual traditions invites a balance between human innovation and moral responsibility. It values scientific progress but also reminds us that such power needs to be guided by respect for human dignity and the common good. From this perspective, the use of gene therapy should not only aim to cure diseases but uphold justice, compassion and equality in access to care. CIT challenges not only researchers but society as well to ensure that technology serves humanity rather than control it, encouraging decisions that reflects both ethical and moral concerns.

(Courtesy of National Human Genome Research Institute)
In closing, gene therapy stands at the intersection of revolutionary science and moral responsibility. Its ability to cure hereditary diseases and transform countless lives, represents humanities greatest medical achievements. Yet, these advancements also demand caution. The development and use of gene therapy should prioritize the moral and the common good. The potential for misuse, unequal access and lack of transparency can loss the main purpose of healing. Ethical concerns include informed consent, fair pricing, and the patient’s safety must remain fair for everyone as gene therapy continues to advance. For this technology to truly serve humanity, it must not only have the scientific precision but with compassion, fairness and integrity, guiding every decision. The question isn’t how far gene therapy can go it’s how accessible it can be and how helpful it can be to the masses.
Citations:
Rubanyi, G. M. (2001). The future of human gene therapy. Molecular Aspects of Medicine, 22(3), 113–142. https://doi.org/10.1016/S0098-2997(01)00004-8
Ansah, E. O. (2022). Ethical Challenges and Controversies in the Practice and Advancement of Gene Therapy. Advances in Cell and Gene Therapy, 2022(1), 1015996. https://doi.org/10.1155/2022/1015996
Fischer, A., & Cavazzana-Calvo, M. (n.d.). Gene therapy of inherited diseases. Retrieved October 8, 2025, from https://www.thelancet.com/journals/lancet/article/PIIS0140673608608740/fulltext
Gonçalves, G. A. R., & Paiva, R. de M. A. (n.d.). Gene therapy: Advances, challenges and perspectives. https://doi.org/10.1590/S1679-45082017RB4024
Kumar, S. R., Markusic, D. M., Biswas, M., High, K. A., & Herzog, R. W. (n.d.). Clinical development of gene therapy: Results and lessons from recent successes. Retrieved October 8, 2025, from https://www.cell.com/molecular-therapy-family/methods/abstract/S2329-0501(16)30177-2
Li, X., Le, Y., Zhang, Z., Nian, X., Liu, B., & Yang, X. (2023). Viral Vector-Based Gene Therapy. International Journal of Molecular Sciences, 24(9), 7736. https://doi.org/10.3390/ijms24097736
The future of human gene therapy. (n.d.).
van Hooff, L. C., Merz, E.-M., Kidane Gebremeskel, A. S., de Jong, J. A., Burchell, G. L., & Lunshof, J. E. (2025). Balancing benefits and burdens: A systematic review on ethical and social dimensions of gene and cell therapies for hereditary blood diseases. BMC Medical Ethics, 26(1), 36. https://doi.org/10.1186/s12910-025-01188-3