Introduction:
The rapid advancement of genetic editing technologies, especially the CRISPR-Cas9 system, has presented a new era in molecular biology and genetics. This tool allows for accurate DNA alterations by utilizing guide RNA to identify specific sequences, coupled with the Cas9 enzyme to make precise cuts in the DNA. This innovative technology empowers scientists to add, remove, or modify genes, paving the way for significant progress in research and medicine. In this new approach, very well-targeted changes can be made to the DNA of any living species, including humans. This provides the possibility of eradicating several diseases at its root caused by “bad” genes. Genetic disorders, such as cystic fibrosis, sickle cell anemia, and Huntington’s disease, might be repaired even at the embryonic stage, securing a healthier future for humanity. This technology has profound implications; both promise enormous benefits and raise significant ethical concerns.
Genetic editing of embryos, eggs, and sperm involves altering genes that could be passed along to future generations. The effects of changes in the germline pose a multitude of ethical and social considerations. Most of the long-term effects are unknown- many scholars, ethicists, and the lay public feel that any benefits of genetic editing do not justify the risk. This critical analysis reviews the promise of genetic editing in eradicating genetic diseases but at the same time, raises the ethical concern with germline modification. Based on an analysis of scholarly research between 2016 and 2024, this manuscript gives an overview of the benefits and risks concerning this new technology. More importantly, it expresses the need for responsible governance and ethics.
The Promise of Genetic Editing:
Scientific Breakthroughs in Genetic Disease Prevention:
The CRISPR-Cas9 technology has revolutionized genetic editing, allowing for targeting and modification at an unprecedented level of accuracy of the specific sections within DNA. This great breakthrough opens an avenue quite strongly toward the correction of harmful mutations responsible for a range of genetic disorders. It was indicated that the “CRISPR-Cas9 technology directly provides the scientist with a means to edit organisms’ genomes, thus opening up new avenues toward possible cures for monogenic disorders-that is, disorders brought about by a mutation in a single gene” 1. Such is the case with cystic fibrosis, a disease of lungs and digestive systems caused by mutations of the gene encoding CFTR. A breakthrough study using human embryos to correct the mutation in the CFTR gene by CRISPR, has shown feasibility for preventing a highly debilitating disease well before birth. The research not only showcased the capability of gene editing but also marked an important step toward eradicating genetic diseases from the Population.
Besides the works on cystic fibrosis, remarkable works are reported by scientists for CRISPR-based treatment of genetic disorders. Recently, a study demonstrated the correction of a mutation in one of the beta-globin genes responsible for sickle cell disease using the CRISPR-Cas9 system 2. In the embryos treated by editing, no sign of this disease was observed; therefore, such germline editing may provide a permanent solution for individuals bearing such mutations. It is this potential for the eradication of diseases that underlines the transformative power of genetic editing in shaping the future of human health.
Possible Benefits: Reducing Human Suffering:
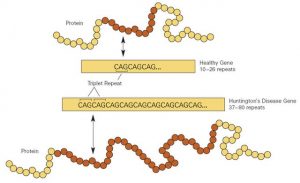
The most critical benefit that speaks to genetic editing is its potential to reduce human suffering at the hands of genetic diseases.Diseases like Huntington’s, with progressive neurodegeneration, may even be completely eliminated by germline editing. In 2020, a study was published in the journal Nature Medicine, where they successfully removed the deleterious alleles responsible for Huntington’s disease from embryos 3. The study not only exhibits the therapeutic capability of CRISPR; rather, germline editing may safeguard future generations from debilitating diseases. Besides, gene-editing technologies would avoid the burden of long-term treatments associated with most chronic conditions. Currently, the treatment paradigm for sickle cell disease consists of frequent hospitalizations and lifelong medications. Using CRISPR-Cas9 to correct the mutation responsible for sickle cell disease, may not only manage the symptoms of the disease but cure it 4. This will not only save a lot of money in healthcare but greatly improve lifestyles by preventing such diseases in the first place.
Minimizing Genetic Risk of Cancer:
It is also an avenue to reduce genetic risks for cancers. Breast and ovarian cancers are alarmingly more common in individuals with mutations in the BRCA1 or BRCA2 genes. In this regard, a study was demonstrated that CRISPR-Cas9 can edit the BRCA1 gene in human embryos and thus avoid passing the mutation to offspring 5. This is a breakthrough in research showing how germline editing could become a crucial tool in reducing cancer risks, by offering preventive healthcare at the genetic level.Beyond its application for hereditary cancer risks, CRISPR finds its extended use in oncology. It is also being researched for the capability of amplifying immune responses against tumors. Researchers are studying whether the CRISPR technology could be applied to immune cells, in order to make them recognize and attach to the cancer cells more effectively. There was an estimation where it was observed that genetically edited T-cells significantly enhanced tumor regression in mouse models of cancers and showed that CRISPR could be used as a strong weapon in the fight against cancer 6. Genetic editing has the potential to revolutionize both inherited and acquired cancer risks, creating new paradigms in cancer prevention and therapy.
Improved Precision, Lower Off-Target Effects:
There are two major concerns related to CRISPR technology safety: the risk of off-target effects, that is, unintended changes in the genome that could have adverse health consequences. Indeed, early versions of CRISPR were less precise, carrying an increased risk of such effects. However, great strides have been made in high-fidelity CRISPR-Cas9 variants that have dramatically improved accuracy. In a review, it discussed how such improvements have minimized the off-target effects of the system, thus making genetic editing both safer and more reliable 7.These newer tools are making it possible to achieve precise edits with few unintended consequences, which should facilitate germline editing applications more toward clinical viability.
A relatively new approach, base editing, allows scientists to change a single DNA base without cutting the DNA strand. This adds more to reducing the risk of errors. It was shown that base editing opens up an avenue for more safety in carrying out traditional CRISPR methods due to the small, precise changes it makes at the molecular level. This reduces off-target effects and generally enhances the safety for gene editing 8. The increased precision that comes with newer methods underlines the promise that responsible genetic editing holds for treating genetic diseases while minimizing risks of off-target mutations.
Opposition to Genetic Editing and Ethical Concerns:
Irreversible Changes and Long-Term Risks:
As much as there is great hope for CRISPR-Cas9 with respect to germline modification, there are those who raise concerns regarding the irreversible effect of genetic alterations. In the case where the modifications touch on the germline, these then become inherited with each subsequent generation. The ramifications of such changes have yet to be known, and many are thus starting to wonder what is morally right about making changes to the human genome. It was observed that not much is known about the long-term risks involved in germline editing, and extensive research is needed before it can be commonly administered 9. Critics say not all genetic mutations are known, and changes in the genome could have far-reaching consequences. Lander, supports the possible health consequences arising from off-target mutations, with a statement that harms can be possible to date; thus, a prudent approach toward germline editing is warranted 10. There are ethical imperatives towards future generations to consider how our actions are going to impact their lives and their health.
Human Dignity and the Fear of Eugenics:

Genome editing raises a myriad of profound and significant questions about human dignity, especially because such knowledge opens avenues to eugenics. It is consequently argued that germline editing will imply a future where some genetic traits are superior to others. This will create a genetic hierarchy, thereby debunking the proposition of equality within humanity. An author, reflected on these issues in her book “Altered Inheritance” and asserts therein that choosing characteristics for “designer babies” commodifies human beings into mere products valuable only by their genetics and not as ends in themselves 11. Such a perspective brings into sharp focus the critical questions of what consequences may be had in making a commodity of human life based on genetic characteristics.
While the social effects of germline editing might only marginally exacerbate pre-existing inequalities, the effects may be more significant; for instance, Sparrow questions the distribution of genetic editing technologies solely among the rich or developed countries 12. This, in itself, will provide a further division, distinguishing those who can, from those who cannot afford to enhance their children’s genes. This new inequality is one where some populations do benefit from enhancements in genetics, while others do not. This only adds onto existing social and economic disparities.
Ethical and Moral Issues:
The ethical and moral issues regarding gene editing have to do with many more aspects than just individual health and the impacts on it. There are a number of ethical theories, to name a few: utilitarianism and deontological ethics, which could be used to analyze the implications of germline modification. Utilitarianism, where an action is said to be right if it encourages the greater good for the largest number of people. This can be used to justify genetic editing if this practice leads to better health conditions and lesser suffering in a larger circle. Critics, however, go ahead to argue that the utilitarian approach may not take into consideration the rights of individuals, especially when one is making permanent changes in their genetic makeup with their consent.
The deontological ethics, on the other hand, which emphasize the intrinsic rights of people, raise concern for the morality of intervening in the human genome. This view asserts that manipulating the genetic code of future generations without their informed consent is inherently wrong. Central to this debate is the ethical principle of autonomy, addressing an individual’s rights regarding one’s own body and health. Discussions related to germline editing need to weigh ethical frameworks carefully, since they shape our understanding of associated rights and responsibilities due to such technologies.
Regulatory and Legal Challenges:
Among the major challenges of germline editing is the absence of robust regulatory frameworks to govern its use. In the United States alone, it is fragmented in regulation, with no comprehensive federal guidelines in place that ensure safety and ethical considerations for germline editing. The lack of clarity in the current legal landscape often leads to inconsistencies in the oversight of gene editing technologies 13. Now, the misuse regarding the same and unethical behavior with a lack of clear regulations is a potential issue.
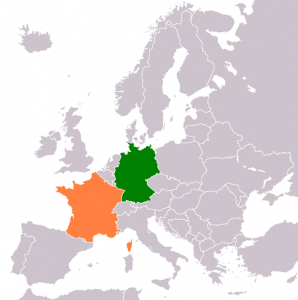
Germline editing has drawn different stances from different countries of the world. The countries like Germany and France have gone as far as putting stern bans on germline modifications, which reflects their cautious approach to the technology. In contrast, most of these countries have taken a more permissive approach; they include China and the United States. This has contributed to quite disparate policies that may poorly address ethical considerations. There is a necessity for international discussion in view of developing consistent measures with a view to safety, ethics, and responsibility regarding research in the field of genetic editing.
Public Engagement and Ethical Discourse:
Public engagement and ethical discourse in this respect go hand in hand, since at stake are issues of great complexity in relation to the task of editing genetic material, with wide implications for society. There is a need for engaging various kinds of stakeholders-scientists, ethicists, policymakers, and the general public-who can facilitate informed discussions on risks and benefits regarding genetic editing. Researchers give prominence to public engagement in shaping the ethical landscape on genetic editing because such a practice can make dialogue meaningful and increase transparency and accountability in research practices 14 Public understanding of genetic editing technologies is better achieved when education informs decision-making. Society is in a position to provide people with accessible information about the underlying science, potential uses, and ethical implications of genetic editing, enabling people to actively participate in discussions on its application. In this light, educational programs at all levels have the potential for helping to dissolve mystique about genetic editing and facilitating a broader public discussion of its social implications.
It is in this light that interdisciplinary collaborations by scientists and ethicists may help in the elaboration of an all-encompassing ethical framework that guides research in the field of genetic editing. As genetic technologies continue to evolve, ethical discourse should be developed hand in hand with scientific progress if societal values are to be protected and respected.
Conclusion:
The promise of genetic editing and germline modifications in eradicating genetic diseases is profound, standing to avert human suffering and improve health outcomes. Breakthroughs in CRISPR technology provide opportunities for the correction of genetic disorders before birth, opening ways toward much healthier future generations. At the same time, one should not underscore the ethical implications of tampering with the human genome. The issue of irreversibility, along with long-term risks and specters of eugenics, gives rise to fundamental questions pertaining to the responsible application of this powerful technology.
The necessary regulatory frameworks and open, informed stakeholder dialogues will be required in order for genetic editing to overcome its challenges. Active involvement in ethics discourses and public engagement should make society realize the benefits of genetic editing with respect for human dignity and social equity. The path to responsible genetic editing is complex, but through open dialogue, we navigate through the minuscule details of this fast-emerging field toward the future in which the promise of genetic editing will be harnessed for the greater good.
- Lander, Eric S. “The Heroes of CRISPR.” Cell, vol. 164, no. 1–2, Jan. 2016, pp. 18–28. PubMed, The Heroes of CRISPR – ScienceDirect. ↵
- Hoban, Megan D., et al. “CRISPR/Cas9-Mediated Correction of the Sickle Mutation in Human CD34+ Cells.” Molecular Therapy: The Journal of the American Society of Gene Therapy, vol. 24, no. 9, Sept. 2016, pp. 1561–69. PubMed, https://doi.org/10.1038/mt.2016.148. ↵
- Men, Ke, et al. “CRISPR/Cas9-Mediated Correction of Human Genetic Disease.” Science China. Life Sciences, vol. 60, no. 5, May 2017, pp. 447–57. PubMed, https://doi.org/10.1007/s11427-017-9032-4. ↵
- Lomunova, M. A., and P. M. Gershovich. “Gene Therapy for Cystic Fibrosis: Recent Advances and Future Prospects.” Acta Naturae, vol. 15, no. 2, June 2023, p. 20. pmc.ncbi.nlm.nih.gov, https://doi.org/10.32607/actanaturae.11708. ↵
- Yang, Haitao, et al. “Break Breast Cancer Addiction by CRISPR/Cas9 Genome Editing.” Journal of Cancer, vol. 9, no. 2, Jan. 2018, p. 219. pmc.ncbi.nlm.nih.gov, https://doi.org/10.7150/jca.22554. ↵
- Azangou-Khyavy, Mohammadreza, et al. “CRISPR/Cas: From Tumor Gene Editing to T Cell-Based Immunotherapy of Cancer.” Frontiers in Immunology, vol. 11, 2020, p. 2062. PubMed, https://doi.org/10.3389/fimmu.2020.02062 ↵
- Kleinstiver, Benjamin P., et al. “High-Fidelity CRISPR-Cas9 Nucleases with No Detectable Genome-Wide off-Target Effects.” Nature, vol. 529, no. 7587, Jan. 2016, pp. 490–95. PubMed, https://doi.org/10.1038/nature16526. ↵
- Graubert, Timothy A., et al. “Recurrent Mutations in the U2AF1 Splicing Factor in Myelodysplastic Syndromes.” Nature Genetics, vol. 44, no. 1, Dec. 2011, pp. 53–57. PubMed, https://doi.org/10.1038/ng.1031 ↵
- Coller, Barry S. “Ethics of Human Genome Editing.” Annual Review of Medicine, vol. 70, Jan. 2019, pp. 289–305. PubMed, https://doi.org/10.1146/annurev-med-112717-094629 ↵
- Lander, Eric S. “The Heroes of CRISPR.” Cell, vol. 164, no. 1–2, Jan. 2016, pp. 18–28. PubMed, The Heroes of CRISPR – ScienceDirect ↵
- Baylis, Françoise. Altered Inheritance: CRISPR and the Ethics of Human Genome Editing. Cambridge, MA and London, England: Harvard University Press, 2019. https://doi.org/10.4159/9780674241954 ↵
- Sparrow, Robert. “Imposing Genetic Diversity.” The American Journal of Bioethics, vol. 15, no. 6, June 2015, pp. 2–10. DOI.org (Crossref), https://doi.org/10.1080/15265161.2015.1028658. ↵
- Vidalis, Takis. “Genome Editing in Human Gametes and Embryos: The Legal Dimension in Europe.” BioTech, vol. 12, no. 1, Dec. 2022, p. 1. pmc.ncbi.nlm.nih.gov, https://doi.org/10.3390/biotech12010001. ↵
- National Academies of Sciences, Engineering, et al. “Public Engagement.” Human Genome Editing: Science, Ethics, and Governance, National Academies Press (US), 2017. www.ncbi.nlm.nih.gov, https://www.ncbi.nlm.nih.gov/books/NBK447279/. ↵

2 comments
Cesia Gonzalez
I love the creativity and originality that you bring to your writing. It is really informative and I really enjoyed reading your piece! 10/10! Great job!
Natalia De la garza
Thank you for sharing this insightful infographic. It effectively illustrates the rapid advancements in genetic editing technologies, particularly the CRISPR-Cas9 system, and their profound implications in molecular biology and genetics.