Introduction
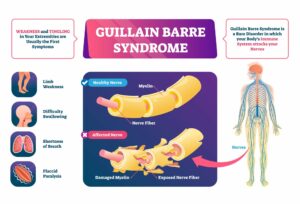
Guillain-Barré syndrome (GBS) is a rare autoimmune disease that causes peripheral neuropathy (disease causing peripheral nerve damage) through an autoimmune mechanism. This involves the immune system mistakenly attacking the nerves. Anyone can obtain GBS, but it is more common in infant and older populations. The exact cause of GBS is unknown, but cases involving GBS are mostly caused by a bacterial infection that produces proteins similar to the proteins the body produces. Rarely, the immune system confuses the proteins made from the body as foreign, thereby triggering an immune response against itself and attacking the nerves in the process.1
During the pandemic, there was evidence that GBS could be caused post COVID-19 infection/vaccination. Several post-acute COVID 19 syndromes (PACS) arose after the pandemic in 2019, and one of these PACS following COVID infection includes Guillain-Barré syndrome.2 Although rare, the mechanism behind how it can occur is unknown, and in this article, I aim to provide understanding on how research efforts are addressing many of the questions surrounding the mechanisms of this disease.
Background (What are other causes of GBS?)
According to the CDC, GBS is the most common in newborns, as 1 in 4 pregnant women carry GBS bacteria in their body, and it can be transmitted to their babies during delivery. Usually, C. jejuni is found in food animals such as poultry, pigs, and cattle. C. jejuni can also be found in household pets such as cats and dogs. However, the main route of infection is through undercooked meat and raw/contaminated milk.3
GBS is most common in adults who are over the age of 65 and have a history of other health conditions including: Diabetes, Heart disease, Cancer, and Obesity.4 Overall, older adults are at an increased risk due to the immune system weakening with age. However, those who live with the conditions above are at an even higher risk because these conditions can further weaken the immune system, making it harder to fight off infections.5 The bacteria/viruses that precede GBS include C. jejuni, Epstein-Barr virus, HIV, Zika virus, and cytomegalovirus.6
Pathogenesis of Guillain-Barré syndrome induced by C. Jejuni
The pathogenesis of GBS is known for its mechanism of molecular mimicry, producing antibodies that target gangliosides.7 Gangliosides are molecules present on nerve cells, which contribute to brain and cognitive development by regulating the growth of nerve cells, of myelin sheaths and synapses.8
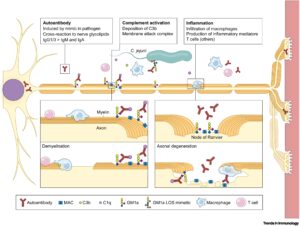
The antibodies secreted are made to be specific for the outer membrane of C. jejuni. However, due to the molecular mimicry of its outer membrane, the antibodies also target the axolemma, degrading the membrane. These kinds of antibodies are called autoantibodies because they target the body instead of the pathogen. This kind of response is rare due to differences between strains of C. jejuni.
Recently, the pandemic affected individuals with not just COVID infection, but also post-acute COVID –19 syndromes (PACS). One of these PACS includes the onset of GBS. An interest in COVID-19 induced GBS was taken when 5 patients developed GBS after COVID-19 infection in early 2020. However, scientists are unsure of how COVID-19 induces GBS.
Proposed Mechanisms of COVID-19 Induced GBS
Above I described the mechanism of action for C. jejuni, but not for COVID-19. The mechanism by which COVID-19 infection induces GBS is poorly understood, and more research needs to be conducted to gain a better understanding of what might be happening. However, the mechanism of C. jejuni provides a basis on how COVID-19 mechanism might work. Few studies have shown theorized mechanisms by which COVID-19 induces GBS.
There are several ways that SARS-COV2 can invade the central nervous system (CNS). It is thought that the virus can reach the CNS by invading the brain through the olfactory nerve (the nerve that allows you to smell) and then spread through trans-synaptic transport, which is the movement of particles across synapses between neurons. Additionally, the virus can pass the blood−brain barrier to enter the central nervous system by migrating through infected leukocytes or after infecting and damaging capillary endothelial cells (cells that line the walls of small blood vessels).12
There are many theories that surround the mechanism of COVID-19 induced GBS. It has been found that the peptides of SARS-CoV-2 proteins are similar to human peptides. Specifically, this similarity was found between two heat shock proteins (HSPs) made within the body (HSP 90 and 60).13 These proteins function as molecular chaperons in cells, by folding and refolding many proteins along with degrading misfolded proteins.14 This finding may suggest that the mechanism mimics C. jejuni induced GBS.
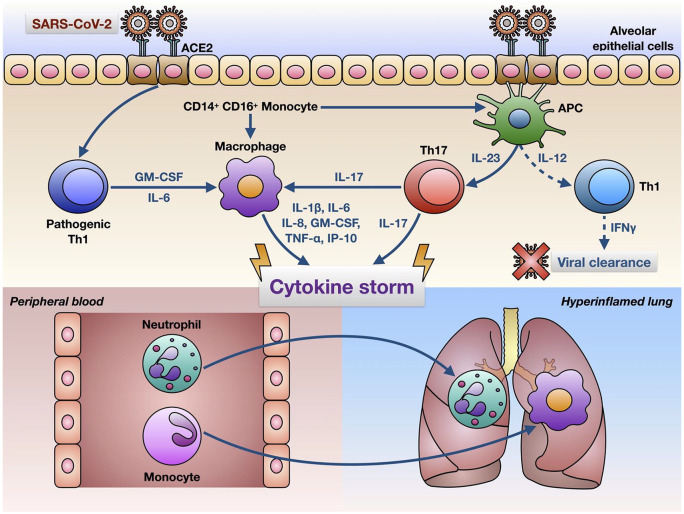
Another hypothesized mechanism involves the production of cytokines, which are signals produced by immune cells to signal to other immune cells to help them mount an immune response. This “cytokine storm” that is proposed to be triggered by COVID-19 is characterized by a surmountable increase in cytokine production. Some of these cytokines include interferon gamma, tumor necrosis factor-α (TNF-α), IL-1β, IL-6, and IL-17 which can induce organ damage to multiple organs. However, it has been noted that IL-6 could be a main determinant of assessing disease severity due to its ability to stimulate an inflammatory cascade.15
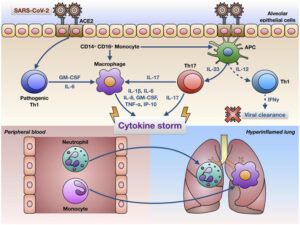
Additionally, there has been evidence that the cytokine storm increases the permeability of the blood-brain barrier, making it easier for pathogens or antibodies to enter the CNS. Cytokine storm can also affect the blood-nerve barrier, resulting in an autoimmune response against the nervous system.
A final theoretical mechanism is that due to immune system hyperactivity induced by SARS-CoV-2 infection, neutrophils form extracellular traps (NETs), which are fibers made up of mainly DNA from the neutrophil which binds to pathogens. Inflammation triggers NET formation, which then causes even more inflammation. Similarly, NETs can act as autoantigens produced during apoptosis (programmed cell death) of the body’s cells in the inflammatory state, leading to autoantibody production, which could possibly lead to GBS18
Diagnosis, Treatment, and Prognosis
Symptoms of GBS present (up to 70% of patients) within 1 to 6 weeks of a previous illness. Usually, patients that have GBS have patterns of proximal and distal weakness which is flaccid. This means that the muscles are weak in various parts of the body, proximal towards the center of the body and distal being in the limbs. Having a flaccid phenotype indicates that the limbs will hang loosely. Another symptom reported is having chronic pain characterized by a prickling or burning sensation in the hands followed by the feet. Many doctors will monitor a patient suspected of having GBS in case the nerves within the respiratory system start to become weak, which means that they will have to be artificially intubated. In up to 30% of patients, respiratory failure can occur which leads to a prolonged hospital stay.19
To confirm a diagnosis of GBS, several tests can be conducted including electrodiagnostic tests, spinal taps, and MRI scans. Electrodiagnostic tests measure the electrical activity of the nerves, which is used to detect nerve damage. Cerebrospinal fluid (CSF) from a spinal tap shows a pattern of albuminocytologic dissociation. This term means that spinal fluid shows a normal amount of white blood cells and an elevated CSF protein level. However, the pattern of albuminocytologic dissociation is only present in 80% of patients at 2 weeks after symptoms present.20
As stated earlier, gangliosides are a part of the membrane of an axon, and many ganglioside antibodies have been associated with GBS. Antibodies secreted against gangliosides include anti-GM1, anti-GD1A, anti-GT1A, and anti-GQ1B. These antibodies differ in sensitivity from up to 60% (anti-GM1 antibodies in acute motor axonal neuropathy) to up to more than 90% (anti-GQ1B antibodies in Miller Fisher syndrome).21
Images using magnetic resonance imaging (MRI) of the spine can show the enhancement of the nerve roots, which would indicate a breakdown of the blood-nerve barrier because of inflammation.22
The two most successful forms of treatment for individuals diagnosed with GBS include intravenous immunoglobulin (IVIG) or plasma exchange. The exact mechanism of IVIG remains poorly understood. However, plasma exchange is thought to work by removing autoantibodies, complement proteins, and other molecules secreted by the immune system that are involved in the pathogenesis of GBS. Similar to IVIG, its exact mechanism of action in the treatment of GBS has not been proven.23
Prognosis for individuals diagnosed with GBS do well. Over 80% of patients are able to walk on their own after six months. The mortality rate for GBS during the acute phase is less than 5%. On the other hand, a subset of those diagnosed with GBS (20%), have disabilities despite obtaining quality care for GBS. For those who survive from GBS, patients may experience continued fatigue, pain, and paresthesias (tingling or prickling sensation in the skin) for several years after diagnosis.24
Conclusion
Overall, GBS is a complex mechanism, which can be triggered by infections of certain pathogens or even immunizations. People who pose an increased risk of GBS are mainly small infants or adults over the age of 65 with other health conditions. Since early 2020 was consumed by the pandemic, minimal resources were able to be allocated towards research of PACS and their long-term effects. Now that the pandemic has been officially declared over, more research is being conducted to better understand COVID-19 induced PACS like GBS. However, since GBS is a rare condition, there is not much opportunity to conduct direct research to uncover the mechanism. All that exists is proposed or theorized mechanisms by which GBS is induced by COVID-19, which indicates that more research needs to be conducted to truly pinpoint how the disease develops.
- People at Increased Risk for Group B Strep | CDC. (2022, November 9). https://www.cdc.gov/groupbstrep/about/increased-risk.html. ↵
- Malekpour, M., Khanmohammadi, S., Meybodi, M. J. E., Shekouh, D., Rahmanian, M. R., Kardeh, S., & Azarpira, N. (2023). COVID‐19 as a trigger of Guillain‐Barré syndrome: A review of the molecular mechanism. Immunity, Inflammation and Disease, 11(5), e875. https://doi.org/10.1002/iid3.875. ↵
- Campylobacter. (n.d.). Retrieved November 1, 2023, from https://www.who.int/news-room/fact-sheets/detail/campylobacter (Campylobacter, n.d.). ↵
- People at Increased Risk for Group B Strep | CDC. (2022, November 9).https://www.cdc.gov/groupbstrep/about/increased-risk.html. ↵
- Group B strep disease-Group B strep disease—Symptoms & causes. (n.d.). Mayo Clinic. Retrieved November 1, 2023, from https://www.mayoclinic.org/diseases-conditions/group-b-strep/symptoms-causes/syc-20351729. ↵
- Abolmaali, M., Rezania, F., Behnagh, A. K., Hamidabad, N. M., Gorji, A., & Mirzaasgari, Z. (2022). Guillain-Barré syndrome in association with COVID-19 vaccination: A systematic review. Immunologic Research, 70(6), 752–764. https://doi.org/10.1007/s12026-022-09316-6. ↵
- Van den Berg et al., B. (2014, July 15). Guillain–Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis | Nature Reviews Neurology. Guillain–Barré Syndrome: Pathogenesis, Diagnosis, Treatment and Prognosis. https://www.nature.com/articles/nrneurol.2014.121/. ↵
- Yamashita, T., Wu, Y.-P., Sandhoff, R., Werth, N., Mizukami, H., Ellis, J. M., Dupree, J. L., Geyer, R., Sandhoff, K., & Proia, R. L. (2005). Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon–glial interactions. ↵
- Laman, J. D., Huizinga, R., Boons, G.-J., & Jacobs, B. C. (2022). Guillain-Barré syndrome: Expanding the concept of molecular mimicry. Trends in Immunology, 43(4), 296–308. https://doi.org/10.1016/j.it.2022.02.003 ↵
- Van den Berg et al., B. (2014, July 15). Guillain–Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis | Nature Reviews Neurology. Guillain–Barré Syndrome: Pathogenesis, Diagnosis, Treatment and Prognosis. https://www.nature.com/articles/nrneurol.2014.121/. ↵
- Guillain Barre syndrome vector illustration. Labeled nerve disease… (2019, May 31). iStock. https://www.istockphoto.com/vector/guillain-barre-syndrome-vector-illustration-labeled-muscle-disease-scheme-gm1152502539-312702423 ↵
- Malekpour, M., Khanmohammadi, S., Meybodi, M. J. E., Shekouh, D., Rahmanian, M. R., Kardeh, S., & Azarpira, N. (2023). COVID‐19 as a trigger of Guillain‐Barré syndrome: A review of the molecular mechanism. Immunity, Inflammation and Disease, 11(5), e875. https://doi.org/10.1002/iid3.875. ↵
- Malekpour, M., Khanmohammadi, S., Meybodi, M. J. E., Shekouh, D., Rahmanian, M. R., Kardeh, S., & Azarpira, N. (2023). COVID‐19 as a trigger of Guillain‐Barré syndrome: A review of the molecular mechanism. Immunity, Inflammation and Disease, 11(5), e875. https://doi.org/10.1002/iid3.875. ↵
- Hu, C., Yang, J., Qi, Z., Wu, H., Wang, B., Zou, F., Mei, H., Liu, J., Wang, W., & Liu, Q. (2022). Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm, 3(3), e161. https://doi.org/10.1002/mco2.161. ↵
- Malekpour, M., Khanmohammadi, S., Meybodi, M. J. E., Shekouh, D., Rahmanian, M. R., Kardeh, S., & Azarpira, N. (2023). COVID‐19 as a trigger of Guillain‐Barré syndrome: A review of the molecular mechanism. Immunity, Inflammation and Disease, 11(5), e875. https://doi.org/10.1002/iid3.875. ↵
- Italy, C. P., Department of Health Sciences, University ‘Magna Graecia’ of Catanzaro, Catanzaro, Calabria, Italy Caterina Tinello, Pediatrics Unit, Provincial Outpatient Center of Catanzaro, Catanzaro, Calabria, Italy Alessandro Vatrella, Department of Medicine, Surgery, and Dentistry, University of Salerno, Salerno, Campania, Italy Giovambattista De Sarro, Department of Health Sciences, University ‘Magna Graecia’ of Catanzaro, Catanzaro, Calabria, Italy Girolamo Pelaia, Campus Universitario ‘Salvatore Venuta’, Viale Europa-Località Germaneto, Catanzaro, 88100. (2021). English: Hypothetical mechanisms underlying the cytokine storm induced by SARS-CoV-2 in infected lungs. (2020). “Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications”. Ther Adv Respir Dis 14: 1753466620933508. DOI:10.1177/1753466620933508. PMID 32539627. PMC: 7298425. https://commons.wikimedia.org/wiki/File:Hypothetical_mechanisms_underlying_the_cytokine_storm_induced_by_SARS-CoV-2_in_infected_lungs.jpg. ↵
- Malekpour, M., Khanmohammadi, S., Meybodi, M. J. E., Shekouh, D., Rahmanian, M. R., Kardeh, S., & Azarpira, N. (2023). COVID‐19 as a trigger of Guillain‐Barré syndrome: A review of the molecular mechanism. Immunity, Inflammation and Disease, 11(5), e875. https://doi.org/10.1002/iid3.875. ↵
- Malekpour, M., Khanmohammadi, S., Meybodi, M. J. E., Shekouh, D., Rahmanian, M. R., Kardeh, S., & Azarpira, N. (2023). COVID‐19 as a trigger of Guillain‐Barré syndrome: A review of the molecular mechanism. Immunity, Inflammation and Disease, 11(5), e875. https://doi.org/10.1002/iid3.875. ↵
- Nguyen, T. P., & Taylor, R. S. (2023). Guillain-Barre Syndrome. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK532254/. ↵
- Nguyen, T. P., & Taylor, R. S. (2023). Guillain-Barre Syndrome. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK532254/. ↵
- Nguyen, T. P., & Taylor, R. S. (2023). Guillain-Barre Syndrome. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK532254/. ↵
- Nguyen, T. P., & Taylor, R. S. (2023). Guillain-Barre Syndrome. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK532254/. ↵
- Nguyen, T. P., & Taylor, R. S. (2023). Guillain-Barre Syndrome. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK532254/. ↵
- Nguyen, T. P., & Taylor, R. S. (2023). Guillain-Barre Syndrome. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK532254/. ↵