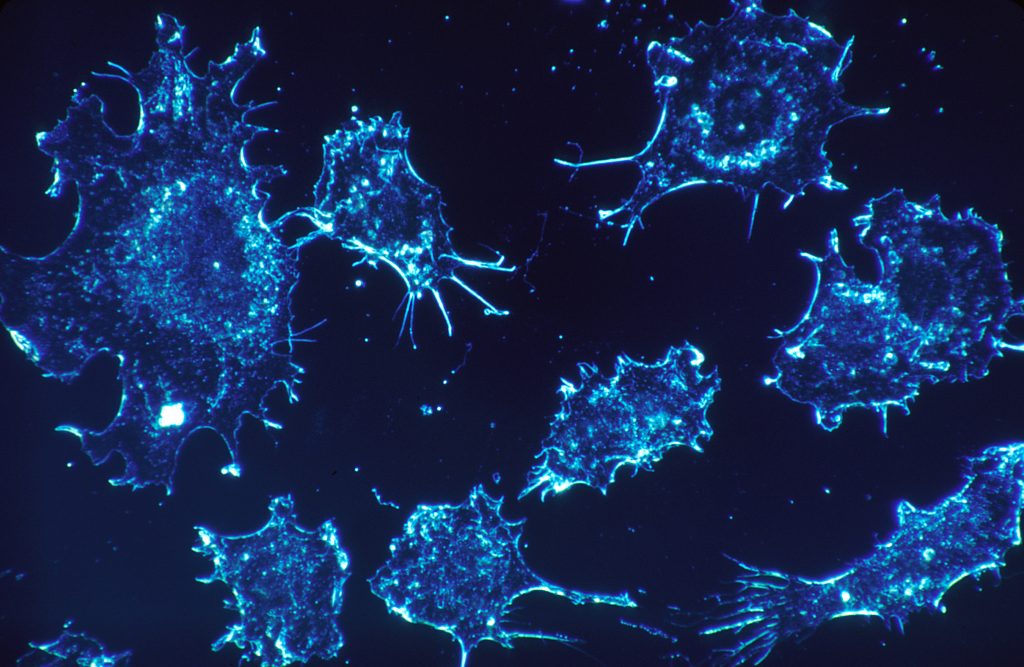
The completion of the human genome in 2003 marked a significant milestone in medical science, laying out the fundamental knowledge needed to understand genetic diseases such as cancer. With the ability to sequence DNA, researchers have been able to identify genetic mutations linked to cancer development, leading to more accurate and earlier detection methods. This advancement has transformed the field of oncology by taking the genomic data of patients and being able to create personalized treatments. Alongside these scientific breakthroughs, many ethical dilemmas have arisen that should be taken into careful consideration. Given that cancer is the second most common cause of death, it is important to understand the risk and prioritize patients’ needs. The American Cancer Society estimated that in 2024, about 2,001,140 new cancer cases and about 611,720 cancer deaths are expected in the United States. It is evident that earlier detection and improved treatments have contributed to a decline in cancer mortality rates since 20211. While the completion of the human genome and advancements in genomic technology have significantly improved earlier and accurate detection of cancer, they also raise critical ethical concerns related to privacy, access to testing, and the potential for overdiagnosis, highlighting the need for a balanced approach to integrating these technologies in clinical practice.
Background into Cancer
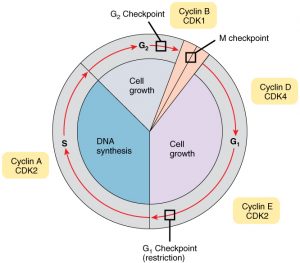
Almost all cells in the body undergo the cell cycle to sustain life, which is where genetic information is retained and passed down to daughter cells in a regulatory manner. The cell cycle consists of three check points which are G1, G2 and M. The G1 checkpoint ensures that the cell has enough energy and is in a favorable environment for DNA replication. The G2 checkpoint occurs before the cell enters mitosis to confirm that DNA has been replicated correctly. Finally, the M checkpoint takes place during mitosis to verify the accuracy of DNA replication2. Cyclins and Cyclin-dependent kinases (CDKs) are two major proteins that regulate the cell cycle. While CDKs are consistently present throughout the cycle, cyclins are produced and degraded at specific times. When either CDKs or cyclins malfunction, it can disrupt other components within the cell cycle that encode tumor suppressor proteins, leading to their inactivity. This disruption can result in uncontrolled cell growth and ultimately lead to cancerous cells3. Although the cell cycle is highly structured and regulated, mistakes can still occur during the transfer of genetic information. Such errors can influence regulatory systems, contributing to the development of cancer when cells proliferate uncontrollably4. In relation to genomics, it’s crucial for cells to proliferate correctly and at a steady rate rather than uncontrollably. While the cell cycle occurs within the nucleus, errors in the DNA transcript can be carried into the cytoplasm that may lead to significant issues. In the cytoplasm, translation takes place, where the DNA information from the nucleus is transported and decoded into RNA. The sequences that are converted into RNA are then translated into amino acid sequences to form proteins. This transition from transcription to translation is collectively referred to as the central dogma of molecular biology, which highlights the flow of genetic information. The proteins are important for various cellular functions, and understanding these processes through a genomic lens helps us analyze how various genes can impact protein production and contribute to diseases such as cancer.
The sequencing of the human genome was completed in 2003 and has provided a basis for cancer research. When comparing normal cells to the genomics of cancer, the study of the complete sequence of DNA and its expression in tumor cells, researchers can explore the mutations leading to cancerous cells, possible treatments, and more evidence to prove past techniques to be correct when identifying cancer5. Through the completed human genome, researchers were able to confirm that past methods used to detect cancerous cells and the approaches taken to find commonly mutated genes within patients experiencing cancer were reliable. For instance, the transformation of cells in culture was a technique used before the human genome was completed. This technique, known as cell transformation, examined how normal cells were converted into tumor cells. In other words, these in vitro techniques showed how cells grew and became cancerous through the causes of radiation, chemicals, and the effects of carcinogenic agents6. A technique that was used shortly after the completion of the human genome was PCR and dye-terminator sequencing. PCR is a technique used to make millions of copies of a specific segment of DNA and dye-terminator sequencing was used to determine the order of nucleotides in a DNA sequence. The researcher, Vogelstein and his team, used this technique to look at genetic changes within the genes of breast and colorectal tumors. Their findings led to identifying “cancer genes” that were commonly found in the cohort they were studying. The mutated genes that were found were APC, TP53, and KRAS in colon cancer and TP53 in breast cancer7. Overall, these findings were determined by using the complete human genome, PCR, and dye-terminator sequencing. These findings also show how the human genome sequence provided a foundation that allowed them to support earlier techniques used in cancer genetics. Overall, there finding matched up with “cancer genes” that we already suspected to be involved in the development of cancer.
Advancements in Technology
With the completion of the human genome and the information we have gathered thus far within the cancer genome, it is evident that advances in genomic technology have contributed significantly to earlier and more accurate detection of cancer. For instance, Next-Generation Sequencing (NGS) is a powerful tool that enables the sequencing of millions of DNA fragments simultaneously, providing detailed insights into the structure of the genome, genetic variations, gene activity, and changes in gene behavior8. In the context of cancer, NGS has played a crucial role in the early detection of the disease. This is particularly important because the sooner cancer is detected in an individual, the more effective the treatment options can be. Whole-Exome Sequencing (WES) is a specific type of NGS that focuses on the coding regions of the genome within a patient. This approach can offer valuable insights into the genetic makeup of tumors and identify various genetic variants that may have contributed to the development of cancer. Furthermore, WES has enhanced targeted sequencing for cancer, making it more accurate and accessible in terms of both time and cost. By utilizing the genomic profiles of patients’ tumors, treatment strategies can be better tailored, resulting in more efficient and less toxic therapeutic options for patients9.
Alongside NGS technology, other methods have provided genomic insights for early cancer diagnosis. Multi-Parameter Flow Cytometry (FCM) is a technique that analyzes genomic features at a single-cell level on a large scale, highlighting cell markers such as intracellular antigens. This method is effective in diagnosing hematological malignancies like hairy cell leukemia by using antibodies that target specific mutations associated with this type of cancer. By incorporating qualitative data from FCM, researchers gain a deeper understanding of cancer behavior, which can inform more effective treatment strategies for patients with this condition. Another method that has arisen from the knowledge of the human genome and cancer genome is Oncotype Dx. Oncotype Dx is a tool used to make personalized risk assessment for cancer patients. By using genomic markers, such as those provided in FMC, oncologists can develop a more effective therapeutic strategy for individual patients. Although this technique was originally used for breast cancer, Oncotype Dx has been adapted for various other cancers. Liquid Biopsy is another technique that has been utilized for early detection of cancer. Unlike traditional methods that involved invasive biopsies, liquid biopsies are non-invasive. When a liquid biopsy is performed, bodily fluids are analyzed to find molecular signatures that are associated with solid tumors. Besides providing early cancer detection, this technique also monitors the progression of cancer in real time. This technique is very important because it runs very little risk when compared to invasive biopsies that were performed before these technological advancements occurred10. With the information that the human genome has provided, it is evident that many technological advancements have been made with the cancer association. Besides the few techniques that have been mentioned, there are many more that could be discussed. Overall, the genome has provided substantial information for researchers to continue growing the cancer genome and advance medicine further in relation to cancer research.
Ethical Issues
Personal Information

Although these techniques can be very beneficial for individuals experiencing cancer, there are significant ethical concerns surrounding the examination of patients’ genomic information. For instance, within the research community, it is very common for data to be shared. This leads to participants in cancer research studies to be worried about their genomic data being used in scientific articles alongside their family history or family tree. This has become a huge ethical concern because many individuals do not want to be identified in these articles. However, to address these concerns, organizations such as the U.S. National Institutes of Health (NIH) have established data-sharing policies aimed at protecting individual participants. These policies require informed consent and involve oversight from Institutional Review Boards (IRBs), ensuring that participants’ privacy and rights are respected in genomic research11.
Overdiagnosis
Another ethical concern is the potential for overdiagnosis. Overdiagnosing a patient can lead to unnecessary anxiety, treatment, and costs. This issue is significant, especially since genetic testing, while potentially cost-effective, can also take a toll on a patient’s life. Although doctors strive for accurate diagnoses, overdiagnosis can sometimes be unavoidable. For example, breast cancer screenings are known to lead to overdiagnosis, which occurs when screenings detect cancers that wouldn’t have caused harm if left undetected. This often happens because current technology struggles to differentiate between aggressive tumors and those that are slow-growing and harmless. Statistically, more than 99% of women diagnosed with cancer through screening undergo surgery. Many face invasive procedures like mastectomies, along with treatments such as chemotherapy and radiation. For women who are overdiagnosed, these interventions provide no real benefit, as they didn’t have a serious condition to begin with. Instead, they bear the physical and emotional burdens of unnecessary treatment, which can include fatigue, lymphedema, and anxiety about their health12. In the end, while breast cancer screening can save lives, it carries the risk of overdiagnosis, which can have lasting effects on women’s physical and mental well-being. Importantly, overdiagnosis is not limited to breast cancer; it can occur in other cancers as well. This highlights the need for careful consideration in cancer screening and the importance of informing patients about the potential risks of overdiagnosis.
Access to Advanced Genomic Technology
Access to advanced genomic technology, such as NGS for early detection and treatment, can be particularly challenging for individuals from lower socioeconomic backgrounds. While insurance is an option for many patients, coverage can be limited. For instance, insurance may not cover the costs of advanced testing or may require prior approval, making it difficult for patients to access these essential technologies13,14. Statistically, about 57% of adults under 65 with a cancer history have employer-based coverage, while 6% purchase insurance through marketplaces created by the Affordable Care Act (ACA). Among patients from lower socioeconomic backgrounds, around two-thirds are covered by Medicaid or Medicare, but about 16% remain uninsured15. Despite the existence of various insurance options, adults under 65 must often rely on employment to secure coverage. This reliance on employment can significantly impact patients’ mental and physical health, especially during ongoing cancer treatment and testing. The stress of maintaining a job while managing health issues can lead to burnout and anxiety. Furthermore, patients may feel compelled to stay in jobs that are not ideal for their well-being, a phenomenon known as “job lock”16. Over time, extensive treatment can lead to unemployment or even early retirement, resulting in loss of insurance and increased financial hardship. This situation highlights the need for more comprehensive support systems that allow patients to focus on their health without the constant pressure of job security. All these ethical concerns come together under the principle of justice in healthcare. Which highlights that all individuals should have equal access to medical advancement especially when dealing with multifactorial disease such as cancer. Since there can be a variety of factors that cause cancer within an individual, these ethical concerns emphasize the right of every individual to have access to these medical advancements.
In conclusion, the completion of the human genome and advances in genomic technology have transformed cancer detection, enabling earlier diagnoses and personalized treatment options. However, these developments also raise important ethical concerns, such as privacy issues, access to testing, and the potential for overdiagnosis. As we move forward in leveraging genomics to combat cancer, it’s essential to prioritize these ethical considerations alongside scientific progress. This balance will help ensure that everyone can benefit from these innovations while protecting their rights and well-being. By fostering an ongoing dialogue among researchers, clinicians, and patients, we can navigate the complexities of genomic medicine and work toward a more effective healthcare system overall.
- Siegel, Rebecca L., Angela N. Giaquinto, and Ahmedin Jemal. “Cancer Statistics, 2024.” CA: A Cancer Journal for Clinicians 74, no. 1 (2024): 12–49. https://doi.org/10.3322/caac.21820. ↵
- Cooper, Geoffrey M. “The Development and Causes of Cancer.” In The Cell: A Molecular Approach. 2nd Edition. Sinauer Associates, 2000. https://www.ncbi.nlm.nih.gov/books/NBK9963/. ↵
- Mercadante, Anthony A., and Anup Kasi. “Genetics, Cancer Cell Cycle Phases.” In StatPearls Publishing, 2023. https://www.ncbi.nlm.nih.gov/books/NBK563158/. ↵
- Mercadante, Anthony A., and Anup Kasi. “Genetics, Cancer Cell Cycle Phases.” In StatPearls Publishing, 2023. https://www.ncbi.nlm.nih.gov/books/NBK563158/. ↵
- Wheeler, David A., and Linghua Wang. “From Human Genome to Cancer Genome: The First Decade.” Genome Research 23, no. 7 (July 2013): 1054–62. https://doi.org/10.1101/gr.157602.113. ↵
- Cooper, Geoffrey M. “The Development and Causes of Cancer.” In The Cell: A Molecular Approach. 2nd Edition. Sinauer Associates, 2000. https://www.ncbi.nlm.nih.gov/books/NBK9963/. ↵
- Piña-Sánchez, Patricia, Antonieta Chávez-González, Martha Ruiz-Tachiquín, Eduardo Vadillo, Alberto Monroy-García, Juan José Montesinos, Rocío Grajales, Marcos Gutiérrez de la Barrera, and Hector Mayani. “Cancer Biology, Epidemiology, and Treatment in the 21st Century: Current Status and Future Challenges From a Biomedical Perspective.” Cancer Control : Journal of the Moffitt Cancer Center 28 (September 27, 2021): 10732748211038735. https://doi.org/10.1177/10732748211038735. ↵
- Satam, Heena, Kandarp Joshi, Upasana Mangrolia, Sanober Waghoo, Gulnaz Zaidi, Shravani Rawool, Ritesh P. Thakare, et al. “Next-Generation Sequencing Technology: Current Trends and Advancements.” Biology 12, no. 7 (July 13, 2023):997.https://doi.org/10.3390/biology12070997. ↵
- Nair, S. Vinod, Madhulaxmi, Gigi Thomas, and Ravindran Ankathil. “Next-Generation Sequencing in Cancer.” Journal of Maxillofacial & Oral Surgery 20, no. 3 (September 2021): 340–44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8313644/. ↵
- Pulumati, Akhil, Anika Pulumati, Bilikere S. Dwarakanath, Amit Verma, and Rao V. L. Papineni. “Technological Advancements in Cancer Diagnostics: Improvements and Limitations.” Cancer Reports 6, no. 2 (January 6, 2023): e1764. https://doi.org/10.1002/cnr2.1764. ↵
- Takashima, Kyoko, Yuichi Maru, Seiichi Mori, Hiroyuki Mano, Tetsuo Noda, and Kaori Muto. “Ethical Concerns on Sharing Genomic Data Including Patients’ Family Members.” BMC Medical Ethics 19, no. 1 (June 18, 2018): 61. https://doi.org/10.1186/s12910-018-0310-5. ↵
- Rogers, Wendy A. “Analysing the Ethics of Breast Cancer Overdiagnosis: A Pathogenic Vulnerability.” Medicine, Health Care and Philosophy 22, no. 1 (March 2019): 129–40. https://doi.org/10.1007/s11019-018-9852-z. ↵
- Chapman, Carolyn Riley. “Ethical, Legal, and Social Implications of Genetic Risk Prediction for Multifactorial Disease: A Narrative Review Identifying Concerns about Interpretation and Use of Polygenic Scores.” Journal of Community Genetics 14, no. 5 (October 1, 2023): 441 52. https://doi.org/10.1007/s12687-022-00625-9. ↵
- Schulmeyer, Carla E., Matthias W. Beckmann, Peter A. Fasching, Lothar Häberle, Henriette = Golcher, Frank Kunath, Bernd Wullich, and Julius Emons. “Improving the Quality of Care for Cancer Patients through Oncological Second Opinions in a Comprehensive Cancer Center: Feasibility of Patient-Initiated Second Opinions through a Health-Insurance Service Point.” Diagnostics (2075-4418) 13, no. 21 (November 1, 2023): 3300. doi:10.3390/diagnostics13213300. ↵
- Yabroff, K. Robin, Joanna F. Doran, Jingxuan Zhao, Fumiko Chino, Ya‐Chen Tina Shih, Xuesong Han, Zhiyuan Zheng, Cathy J. Bradley, and Monica F. Bryant. “Cancer Diagnosis and Treatment in Working‐age Adults: Implications for Employment, Health Insurance Coverage, and Financial Hardship in the United States.” CA: A Cancer Journal for Clinicians 74, no. 4 (July 2024): 341–58. https://doi.org/10.3322/caac.21837. ↵
- Yabroff, K. Robin, Joanna F. Doran, Jingxuan Zhao, Fumiko Chino, Ya‐Chen Tina Shih, Xuesong Han, Zhiyuan Zheng, Cathy J. Bradley, and Monica F. Bryant. “Cancer Diagnosis and Treatment in Working‐age Adults: Implications for Employment, Health Insurance Coverage, and Financial Hardship in the United States.” CA: A Cancer Journal for Clinicians 74, no. 4 (July 2024): 341–58. https://doi.org/10.3322/caac.21837. ↵