
Eighteenth-century Paris, where the streets were echoing with the sounds of the Enlightenment; the Royal Academy of Science in Paris stood as the last monument of an almost forgotten time, serving as a network of discourse where scientists would present their ideas and theories subjecting them to discussions, all the while having to defend their ideas with evidence and arguments. Even with a newfound pursuit of knowledge brought by the Enlightenment, alchemy reigned supreme as the chosen chemistry that common folk and scholars alike followed. Between the scientists of the Enlightenment and the chemistry of the ancient Greeks stood a barrier of time. Much of the alchemist’s effort went into decoding information that would be questionable if it ever could be understood. Alchemists viewed alchemy as a religious philosophy that would answer the questions of the universe when understood. For them, the laboratory had no role in this.1 However, there stood a man who viewed the world with the purpose of uncovering its mysteries, seeing past what he was told to believe: Antoine Lavoisier. This was a man whose very existence ignited the spark of the chemical revolution.2 The issue that faces any revolution is those who oppose it, whether it is because they choose to or because they know nothing else. Lavoisier knew many would oppose him simply because he suggested experiments as the future of chemistry. He had to find supporters for his revolution, but the chemists of the time were in bitter opposition to Lavoisier.3

In an effort to change the minds of his fellow chemists, Lavoisier tackled the leading alchemy theory of combustion. This theory supported the belief that materials contained a burnable substance called phlogiston. This would be found in great quantities in the atmosphere and in certain materials, making them flammable, thus leading the materials to combust. And when the phlogiston ran out, the material stopped burning.4 In other words, materials themselves cannot burn, but rather the positive phlogiston is what allows things to burn, and the quantity they contain dictates how easily it can be burned. This was the main crux of alchemy: the combustion and chemical reactions that followed the burning of certain metals and items. When the air became too saturated with negative phlogiston, then it was no longer possible to burn something, since there was no more room for it to exist in.
Lavoisier, a firm believer in experimenting, burned organic materials to test his own theory. He weighed and collected data that directly correlated to the idea that there has to exist some other substance in the air that allowed for materials to combust. He called this unknown substance that was found in the atmosphere, oxygen. The leading chemist of the provinces, Guyton de Morveau of Dijion, had directly countered Lavoisier’s oxygen theory, stating that “phlogiston possessed the spirit of levity,” and he outright refused to acknowledge oxygen as a valid theory. Nowhere did there exist a chemist who scrutinized Lavoisier’s theories more than Guyton de Morveau.5 This scrutinization extended beyond Lavoisier’s peers, with figures such as Arthur Young, who went as far as to rank Guyton de Morveau as “not only the first chemist of France, but one of the greatest that Europe has to boast.” This was a rank that historically was never given to Guyton, mostly due to Lavoisier being seen as the genius of this time period. In spite of the opposition, Lavoisier remained determined, not only to prove his theories through experimentation, but also to show other skeptic chemists the future of chemistry. As such, Lavoisier focused his efforts on convincing his biggest doubter, Guyton de Morveau. He did this by performing meticulous experiments, documenting his research, and giving Guyton de Morveau a tour of his laboratory. This had the effect Lavoisier was looking for. De Morveau entered as Lavoisier’s biggest critic and left supporting him.6
This, however, would not be enough to convince the Royal Academy of Science in Paris to leave alchemy behind and stand behind Lavoisier. Knowing this, Lavoisier decided to delve deeper into his theory of oxygen to mount an attack on phlogiston and prove that “Stahl’s phlogiston is imaginary.”7 This was a long process since Lavoisier still needed to figure out exactly what oxygen was. So he started experimenting. August 1774, Joseph Priestley had discovered that by burning mercury calx and collecting the “pure air” produced, candles burned more vigorously. He then presented his theory of “dephlogisticated air” to Lavoisier. Upon hearing this, Lavoisier decided to test it out himself using mercury and other metal calces. After testing different materials, Lavoisier argued that there had to be more to common air than this. There had to be some component that allowed for respiration, and another that asphyxiated. Five years later, Lavoisier was ready to present to the Royal Academy of Science in Paris his theory that acids contained breathable air, and that combustion had to be through a reaction of a substance with that air that he called oxygen. One issue with this theory was that oxygen is not an acid former. In an experiment in June of 1783, Lavoisier realized this when the union of oxygen and common air produced water, which was definitely not an acid. Even with this setback, Lavoisier knew there had to be more to his theory, and to prove that, he decided to pivot, focusing more on water to try to understand how exactly water was formed.
Later that same month and year, in June of 1783, Lavoisier used inflammable air, which is a highly combustible gas, and reacted oxygen with it, producing water. From this, he reasoned that water could not be an element since it could be broken down into simpler parts, oxygen and hydrogen. By decomposing water into these two separate gasses, Lavoisier proved that water was not an element. And now he had all the evidence he needed to disprove phlogiston. By combining this newfound decomposition of water and thus proving that oxygen had indeed been what the other chemists of his time had been proving, Lavoisier was building his argument. This alone, however, would not be enough to convince other chemists, much less the Royal Academy of Science in Paris, that alchemy was wrong, since both the synthetization and decomposition of water could still be explained by the phlogiston theory. However, if Lavoisier could somehow prove that oxygen is what allowed for combustion to occur, and that water was created as a byproduct of oxygen, then maybe, just maybe he stood a chance against centuries of tradition. 8
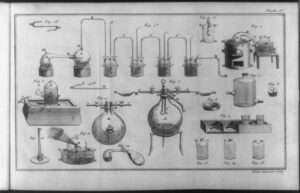
In order for Lavoisier to deliver the final blow to alchemy, he needed to ensure the experiment he chose had not only a well-thought-out method, but also allowed for repeatability. This would make it harder for other chemists to disprove his experiment and simultaneously allow them to try the experiment themselves. Lavoisier decided to light a candle in a jar where he would control the air in the jar and be able to weigh the gasses and mass. This would prove that if phlogiston was real, then the mass should decrease while the gas weight increased rapidly as negative mass phlogiston filled the container. The setup for the experiment was designed to be a controlled environment with little chance for unknown variables to interfere. So the candle was placed in a specialized sealed glass jar, allowing for both observation and measurements to be recorded before, during, and after combustion occurred. As the candle burned, changes immediately started occurring. The candle’s mass started decreasing due to the loss of wax to heat. While the wax burned, Lavoisier noted that water vapor started to appear on the inside of the jar container, hinting at a conversion process that had to do with combustion and air. It wasn’t until Lavoisier checked the gas analysis that he realized the changes in gas volume directly correlated to the increase of water vapor, decreasing as more water vapor formed. By isolating the combustion, Lavoisier could directly observe the role that air played within the jar and prove that phlogiston could not explain why the weight of the candle decreased nor the creation of the water vapor. 9
According to the oxygen theory, combustion is the process in which oxygen is consumed by a burning material that keeps the material burning. Without oxygen, nothing can burn, since there is no oxygen that can be used up in the reaction to sustain fire. A candle is made up of wax, which is a hydrocarbon compound. Simply put, this means that it contains both carbon and hydrogen. When the heat from the fire melts the wax, it releases those hydrocarbon molecules in the oxygen-rich air, reacting and producing both carbon dioxide (CO2) and water (H2O). The formation of the water vapor occurred when the heat of the fire combusted the hydrogen molecules, allowing them to bond to the oxygen. This is why the weight of the gasses was decreasing, because the gasses had combined and made water vapor, which eliminated oxygen that was now sticking to the side of the jar. As the hydrocarbon wax was melting, it lost mass, since it was being evaporated and converted to gasses. However, according to the phlogiston theory, the candle wax is a material that contains phlogiston, which is what sustains combustion. As the candle wax would burn, the phlogiston would then start to be used up and stay in the jar as a negative phlogiston, meaning that it could no longer be used in combustion. This would have the same outcome as the oxygen theory, that there is a finite point where a material can no longer be burned, since there is nothing left to sustain the fire. The issue with the phlogiston theory is that the mass of the gasses should have increased as more phlogiston started to fill up the container directly correlating to the loss of mass of the candle. As this did not happen, there was no quantitative data that could back up the existence of phlogiston. Meanwhile, oxygen had more and more evidence in favor of it.10
There was just one thing left before Lavoisier could be cemented as the Father of Modern Chemistry: the Royal Academy of Science in Paris had to approve his experiment. This meant the candle experiment had to undergo intense scrutiny by not only chemists in France but all over the world, such as by chemists in England and Germany. Thanks to the rigorous experimentation that Lavoisier had performed, no chemist could disprove his oxygen theory. As such, it became accepted by the scientific community. While this was the first nail in the coffin for alchemy as a science, what hastened the death of alchemy was the creation of nomenclature that Lavoisier proposed with the help of Guyton de Morveau and others. Nomenclature is the naming system given to chemical structures, and Lavoisier based this system on his discovery of oxygen. In other words, oxygen is what allowed Lavoisier to create a new nomenclature that would replace the old alchemy system, so much so, that if the theory of oxygen were wrong, the whole system would have fallen with it.11 Even though many had a distaste for Lavoisier, even German scholars, who considered themselves the chemical teachers of Europe, had to accept Lavoisier’s genius and his new theory. Joseph Black, one of the greatest chemists in Germany, adopted all of Lavoisier’s doctrines.
Lavoisier not only disproved alchemy and made chemistry into a true science, but his groundbreaking experimentation on combustion and the discovery of oxygen made chemistry what it is today. Lavoisier had forever changed the world, and with his advancement of nomenclature based on his theory of oxygen completely and irreversibly transformed the scientific world. Therefore, there is no doubt that Lavoisier is truly the father of modern-day chemistry and took chemistry beyond alchemy.
- Madison Smartt Bell, Lavoisier in the Year One: The Birth of a New Science in an Age of Revolution, 1. Ed, Great Discoveries (New York: Norton u.a., 2005), 30-35. ↵
- C. E. Perrin, “Research Traditions, Lavoisier, and the Chemical Revolution,” Osiris 4 (1988): 79. http://www.jstor.org/stable/301743. ↵
- Sidney French, “Torch and Crucible: The Life and Death of Antoine Lavoisier,” In Torch and Crucible: The Life and Death of Antoine Lavoisier (Princeton University Press, 1941), 104. ↵
- Ellen Bailey, “Antoine Lavoisier,” Antoine Lavoisier, Great Neck Publishing August 1, 2017, 1–3 . ↵
- Sidney French, “Torch and Crucible: The Life and Death of Antoine Lavoisier,” In Torch and Crucible: The Life and Death of Antoine Lavoisier (Princeton University Press, 1941), 168. ↵
- Maurice Crosland, “Louis Bernard Guyton de Morveau | French Chemist, Educator & Revolutionary,” Britannica, March 11, 2024, https://www.britannica.com/biography/Louis-Bernard-Guyton-de-Morveau. ↵
- Arthur F. Scott, “The Invention of the Balloon and the Birth of Modern Chemistry,” Scientific American 250, no. 1 (1984): 133. http://www.jstor.org/stable/24969284. ↵
- Antoine Lavoisier, Traité Élémentaire de Chimie, tomes 1 & 2, vol. (Royal Academy of Science and Royal Society of Medicine, 1787), 87-102 ↵
- Antoine Lavoisier, Traité Élémentaire de Chimie, tomes 1 & 2, vol. (Royal Academy of Science and Royal Society of Medicine, 1787). 366-370. ↵
- “Combustion – Chemical Reactions, Heat, Oxidation,” Britannica, accessed April 4, 2024, https://www.britannica.com/science/combustion/History-of-the-study-of-combustion. ↵
- Sidney French, “Torch and Crucible: The Life and Death of Antoine Lavoisier,” in Torch and Crucible: The Life and Death of Antoine Lavoisier (Princeton University Press, 1941), 174. ↵




10 comments
Jordan Robbins
Hi, through your writing I learned so much about Antoine! I never even heard of this name before but its interesting to know how much influence they have on modern science today! Thank you for shedding light on their story and their influence in Chem.!
Carlos Anthony Alonzo
Congratulations on your research! Your study on Antoine Lavoisier is a significant contribution to his story, offering valuable insights into his effort in science. Your methodology is robust, and your findings are well-supported by evidence. I appreciate the clarity of your presentation and the depth of your analysis. Your work sets a high standard for future research in this area. Great job!
Lauren Sahadi
I’ve never heard of Antonione Lavoisier, so it was really interesting to read about someone new. He played a big role in the transition to alchemy to modern chemistry. This article was really great at showing his contributions and the challenges he faced. I love chemistry, so I really enjoyed this article. I’m surprised I’ve never heard of him before. Overall, great article.
Fernando Milian
This article about Antoine Lavoisier was really interesting! It showed how Lavoisier challenged the old ideas of alchemy with his new theories about oxygen, which totally changed how people understood chemistry. I thought it was cool how he didn’t just accept what everyone else believed and instead did his own experiments to prove his points. It’s inspiring to see someone stand up for what they believe in, especially when almost everyone else disagreed with him. It really shows how asking questions and testing things out can lead to big discoveries.
Joseph Reed
I greatly enjoyed reading this article, as it’s a story I had never heard of before; of course, many know of alchemy from fantasy media and history and the like, but it was certainly interesting to see how hard it was to bring down as a science.
Luis Ramirez
Great job! I like the way you demonstrate Antoine Lavoisier’s unwavering dedication to experimentation and his revolutionary discoveries in chemistry which marked the definitive shift from alchemy to modern science. I want to say you did an awesome job when it came to Lavoisier’s contributions. He not only reshaped scientific understanding but also laid the foundation for modern chemistry, ensuring his legacy as the father of the discipline.
Sebastian Hernandez-Soihit
Very helpful and appropiate visual depictions and well sourced documentation about this exciting historical period. Really thought-provoking and easily puts things into perspective with today about things that we perhaps take for granted; we are often thought the science but not the people who were behind it!
Sebastian Hernandez-Soihit
Great Article! Very helpful images and well sourced documentation about this exciting historical period. Really thought provoking and easily puts things into perspective with today about things that we perhaps take for granted; we are often thought the science but not the people who were behind it!
Austin Rolirad
That was very interesting! The many details and the way you put everything together was very engaging and all the facts were well explained. I enjoyed learning about Lavoisier and his advancements in chemistry and alchemy as well as the struggles he went through and how he overcame them, but I must say it was the phlogiston theory and how he used it in his work that caught my interest the most.
Quinten Mero
Great job! As someone not scientifically inclined, I found your explanations of Lavoisier’s scientific research and discovery easy to understand and engaging. Lavoisier’s story of becoming the “Father of Modern Chemistry,” is a great example of the rewards of determination in the face of resistance. I’m sure that without Mr. Lavoisier’s efforts to usher in a new era the world today would look much different.