Introduction
Pharmacogenomics, often called PGx, studies how a person’s genes affect their response to medications, but at its core, it’s about understanding why treatments work differently for real people sitting across from their doctors(Roden et al. 2011; Abe 2016). By linking genetics with drug safety and effectiveness, it offers a more personalized approach to healthcare. For example, certain genetic variants can explain why one person reacts well to an antidepressant while another experiences side effects(Mattes 2004). This field is exciting because it connects what we know about DNA with practical treatment decisions. However, as with any new technology, pharmacogenomics raises ethical questions. Physicians now face choices about how to interpret genetic data responsibly, how to protect patient privacy, and how to make sure everyone has fair access to testing (Burke and Psaty 2007). Before diving into the genetic
science, it’s important to understand what makes pharmacogenomics different from other lab tests, it doesn’t just reveal how a drug works, it reveals something deeply personal about the person taking it. Using Bernard Lonergan’s framework of the four transcendental imperatives, being attentive, intelligent, reasonable, and responsible, this paper looks at how pharmacogenomics challenges clinical judgment. The Catholic Intellectual Tradition adds another layer by reminding us, that human dignity and justice should guide all medical decisions, especially when they involve something as personal as our genes.
Genetic Foundations of Pharmacogenomics
Pharmacogenomics focuses on how variations in genes influence drug metabolism and response. Genes like CYP2D6, CYP2C19, and VKORC1 are commonly tested because they help determine how quickly or slowly a person processes certain medications. For instance, someone with a non-functional CYP2C19 variant might metabolize antidepressants more slowly, which can lead to higher drug levels and unwanted effects (Roden et al. 2011; Caudle et al. 2013). Similarly, differences in the SLCO1B1 gene can affect how statins are processed in the liver and whether patients experience muscle pain. These discoveries help doctors move beyond trial-and-error medicine, sparing patients from months of frustration or dangerous side effects. Every genetic variant represents a chance to make a treatment feel more personal, and more human.
Understanding these genetic patterns helps clinicians move away from “one-size-fits-all” prescribing. But even as pharmacogenomics becomes more common, it also reveals how complicated human biology is. No single gene determines how a drug will act; environment, diet, other medications, and even stress can change the outcome. In this sense, being attentive, means paying close attention to both the genetic data and the context of each patient (Pirmohamed 2011). The science matters, but so does empathy and awareness of the person behind the test result. This blend of data and empathy becomes even more critical when physicians start interpreting what those genes actually mean for treatment decisions.
Interpreting Genetic Information and Ethical Tensions
As pharmacogenomic testing grows, physicians must interpret data carefully and ethically. This part of Lonergan’s framework, being intelligent, requires understanding what genetic information really means. For example, a TPMT gene test can identify patients who would react badly to thiopurine drugs used in leukemia or autoimmune diseases. Another example is the HLA-B gene, which can predict dangerous allergic reactions to drugs like abacavir (Caudle et al. 2013). These insights save lives, but they also raise new questions: Should every patient be tested before taking these medications? Who decides which tests are necessary, and who pays for them?
The promise of pharmacogenomics also comes with anxiety. For instance, direct-to-consumer genetic testing companies like 23andMe partnering with pharmaceutical firms, sparked debates about who owns that data. Many patients wonder what happens once their DNA is on file, who sees it, who profits from it, and whether it might one day be used against them. There are also concerns about what happens to a patient’s data after testing. Pharmacogenomic results are often stored in electronic health records, which raises issues about privacy and ownership. If this information is shared too widely, it could be used in ways patients never agreed to, by insurance companies, employers, or research databases. Physicians need to explain these risks in clear language so patients can give informed consent (Beauchamp and Childress 2019).
The Catholic Intellectual Tradition emphasizes using knowledge for the common good. That means scientific progress should never come at the expense of trust or human dignity. Physicians must interpret genetic information not only with technical skill but also with humility and moral awareness. Ethical decision-making in pharmacogenomics isn’t just a clinical process, it’s relational. Physicians hold a position of trust, and how they discuss genetic results can shape a patient’s entire view of medicine. If patients sense that their concerns are dismissed, they may reject testing altogether, even if it could help them. The tone of a conversation, the willingness to explain uncertainty, and the effort to maintain transparency are all part of ethical practice. Building trust ensures that technology supports healing rather than fear, turning a potentially intimidating genetic result into an opportunity for empowerment.
Ethical Judgment in Clinical Practice
Being reasonable means exercising sound judgment about when and how to use pharmacogenomic information. Some PGx tests are extremely helpful, like HLA-B testing before carbamazepine prescriptions, which can prevent life-threatening skin reactions. Others, like some antidepressant panels, are still being studied and might not always change outcomes. Physicians must decide when a test is truly necessary and when it adds little value.
Beyond technical interpretation, clinicians also face moral choices that affect entire communities. Another issue is fairness. Pharmacogenomic data often come from people of European ancestry, so the results may not apply equally to other populations. Without diverse research, precision medicine risks leaving many patients behind (Pirmohamed 2011). Cost is another barrier: PGx testing can be expensive, and not all insurance plans cover it. From an ethical and Catholic perspective, these inequalities challenge the idea of justice in healthcare. A reasonable healthcare system should ensure that all people, regardless of background or income, can benefit from medical innovation. These inequities are not just statistical, they’re personal. They mean that someone’s grandmother might get an inaccurate test result simply because her ancestry isn’t represented in the data. Ethical medicine demands that no one’s biology makes them invisible.
Finally, clinicians must avoid genetic reductionism, or the belief that genes alone determine health. A patient labeled a “poor metabolizer” might feel stigmatized or anxious. Doctors should communicate clearly that genetic variation is just one factor among many that influence treatment response. Judgment guided by reason involves honesty, balance, and compassion (Catholic Bishops, 2016). Ultimately, ethical judgment in pharmacogenomics must extend beyond individual patient encounters. Institutions themselves carry moral weight in how they design consent forms, allocate testing resources, and prioritize research funding. A hospital guided by Catholic social teaching would emphasize solidarity, making sure discoveries benefit marginalized groups and not just those who can afford them. By shaping policies that reflect compassion and justice, healthcare organizations can transform individual ethical choices into systemic change.
Responsibility and Moral Action
Being responsible is where ethics and practice meet. Physicians who use pharmacogenomic testing must take responsibility for how they communicate, protect, and act on this information (Beauchamp and Childress 2019).
Informed consent is a key part of responsibility. For many patients, being asked to share their genetic information feels deeply intimate. It’s not just another lab test; it’s a glimpse into who they are. For some patients, this can bring relief and hope, but for others, it may feel invasive or even frightening. A responsible physician recognizes this emotional layer as part of ethical care. Patients deserve to know what a test will measure, what its limitations are, and how the results might affect their care. This conversation should be interactive and respectful, not rushed or overly technical. Patients should feel empowered to ask questions and decide whether they want the test.
Responsibility also includes protecting privacy. Once genetic information enters a medical record, it must be safeguarded. Breaches could cause emotional or financial harm, especially if the data were used to discriminate. Ethical responsibility means handling this information as something deeply personal, not as ordinary lab data.
Finally, clinicians have a responsibility to advocate for equity. The benefits of pharmacogenomics should not be limited to major hospitals or wealthy patients. Expanding research participation and improving access to testing aligns with Catholic values of justice and solidarity. True responsibility means seeing patients not as sources of data but as partners in discovery. Medicine grounded in responsibility strives to serve everyone, especially those historically left out of scientific progress (Catholic Bishops, 2016).
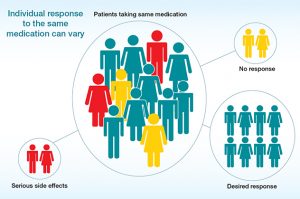
Case Example: Applying Ethical Pharmacogenomics
To see how these ethical principles play out in real life, consider a clinical example where genetics and moral judgment intersect. A helpful example is carbamazepine use in patients of Asian descent. Research has shown that people with a certain allele face a high risk of Stevens-Johnson syndrome, a severe skin reaction. In this situation, a clinician who orders a genetic test before prescribing the drug is acting both scientifically and ethically. If the patient tests positive, the physician can choose a safer alternative, discuss the results openly, and document the findings responsibly (Caudle et al. 2013).
This example shows how the four imperatives can guide real practice: attentiveness to the genetic risk, intelligence in interpreting the data, reasonableness in explaining options, and responsibility in following through with safe care. It also reflects Catholic teaching on human dignity and the duty to use knowledge in service of life. In situations like this, science and compassion meet. The physician’s decision isn’t just about following a guideline, it’s about protecting a life and building trust through honesty.
Integrating Faith, Science, and Justice
The Catholic Intellectual Tradition views science and faith as complementary rather than opposing. In pharmacogenomics, this means using scientific discoveries to serve people rather than control them. The human genome is part of God’s creation, and studying it responsibly honors that gift. This idea reframes scientific inquiry as a form of stewardship. In Catholic thought, using knowledge to heal rather than exploit, mirrors the moral call to care for creation itself. By grounding medicine in faith, clinicians not only apply ethics, but they also practice vocation. When clinicians apply Lonergan’s framework, they practice self-awareness: staying open to evidence while remaining grounded in moral values (Catholic Bishops, 2016).
Being attentive and intelligent helps doctors recognize the beauty and complexity of life at the molecular level. Being reasonable and responsible ensures that this knowledge is used for healing rather than harm. Together, these virtues create an ethical framework where precision medicine can flourish without losing sight of compassion.
Conclusion
Pharmacogenomics has incredible potential to make medicine safer and more effective, but it also challenges how clinicians think about responsibility and ethics. By combining genetic knowledge with Lonergan’s four imperatives and the values of the Catholic Intellectual Tradition, healthcare professionals can navigate these challenges with integrity.
To be attentive is to see the patient as a whole person, not just a genome. To be intelligent is to interpret data with care and humility. To be reasonable is to weigh benefits and harms fairly. And to be responsible is to act in ways that protect dignity, privacy, and justice. When medicine unites science, ethics, and faith, it fulfills its highest purpose, to heal with wisdom and love. Pharmacogenomics will keep advancing, but its success will always depend on how we treat the people it serves. The most responsible medicine listens, to data, yes, but also to stories, fears, and hopes. This balance, between intellect and empathy, science and soul, is what transforms pharmacogenomics from a technical tool into an act of love and justice.
References
Abe, Hiroyuki. 2016. “New Era in Personalized Medicine.” Personalized Medicine Universe 5 (July): 1–2. https://doi.org/10.1016/j.pmu.2016.04.001.
Beauchamp, Tom, and James Childress. 2019. “Principles of Biomedical Ethics: Marking Its Fortieth Anniversary.” The American Journal of Bioethics 19 (11): 9–12. https://doi.org/10.1080/15265161.2019.1665402.
Burke, Wylie, and Bruce M. Psaty. 2007. “Personalized Medicine in the Era of Genomics.” JAMA 298 (14): 1682–84. https://doi.org/10.1001/jama.298.14.1682.
“Catholic Bishops.” n.d.
Caudle, K E, C F Thorn, T E Klein, et al. 2013. “Clinical Pharmacogenetics Implementation Consortium Guidelines for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing.” Clinical Pharmacology and Therapeutics 94 (6): 640–45. https://doi.org/10.1038/clpt.2013.172.
Mattes, William. 2004. “Database Development in Toxicogenomics: Issues and Efforts.” https://doi.org/10.1289/ehp.6697.
Osmosis. 2025. “Genomics – Pharmacogenomics: Nursing: Video & Causes.” Accessed November 6, 2025. https://www.osmosis.org/learn/Genomics_-_Pharmacogenomics:_Nursing.
Pirmohamed, Munir. 2011. “Pharmacogenetics: Past, Present and Future.” Drug Discovery Today, Special Issue: Pharmocogenetics, vol. 16 (19): 852–61. https://doi.org/10.1016/j.drudis.2011.08.006.
Roden, Dan M., Russell A. Wilke, Heyo K. Kroemer, and C. Michael Stein. 2011. “Pharmacogenomics: The Genetics of Variable Drug Responses.” Circulation 123 (15): 1661–70. https://doi.org/10.1161/CIRCULATIONAHA.109.914820.



