Introduction
The Amazon, known to most as the “lungs of the Earth,” is under threat as the palm oil business grows and forever changes this forest’s rich, yet fragile ecosystem. With uses ranging from cosmetics, to food, and even to biofuels, the demand for palm oil is increasing, leading to vast amounts of deforestation to make space for plantations1 The immense and extraordinary Amazonian rainforest, spanning across nine South American countries and over 5 million km2, is home to an incredible diversity of plants and animals2 Undeniably, however, the Amazon is now under threat, primarily due to deforestation. The devastating effect of this industry-driven mayhem is reshaping and negatively affecting the biodiversity of the rainforest, and many species of plants and animals are losing their habitat and facing the risk of extinction. Moreover, deforestation due to the expansion of palm oil plantations reduces the ability of the Amazon to store atmospheric carbon dioxide, leading to an enhanced greenhouse effect, which in turn contributes to global warming and climate change. For these reasons, all conservation efforts in the Amazon, large or small, are of importance. Prioritizing the search for sustainable ways to produce palm oil is critical to ensure the long-term preservation of this and other rainforests. Failing to do so may result in the Amazon reaching an irreversible tipping point from which it may never recover. And one we may all regret.
The Dawning of Change: The Introduction of Plantations in the Amazon
Few places in the world are so well-known as the Amazon Rainforest. Besides its huge expanse, this old-growth tropical forest is home to at least one-fourth of the entire planet’s terrestrial species. 3 Its biodiversity and dynamic ecosystems are both irreplaceable and without equal. To understand the threats the Amazon now faces, however, and propose solutions to safeguard it, it is important to also understand why it became subject to deforestation in the first place. As is no surprise, an increase in rural population densities and economic incentives for a growing number of cultivators] were the initial deforestation driving forces. The two decades following World War II were known for political turmoil, but also technological breakthroughs.4 The Cold War extended to the Americas, and along with post war decolonization and the Cuban revolution, insurrections in rural areas rapidly began to operate from deep within forests.5 Government officials in Latin America often insisted on agrarian reforms and attractive programs that could provide farming lands for revolutionaries to reduce their engagement in political activities.6
In the second half of the 20th century, Brazil, which holds the majority of the Amazon rainforest, pledged to bring “people without land” to the “land without people”. Penetration roads were built and settlements for people who supported the government were established along these roads and the new Trans-Amazon Highway.7 Moreover, feeder roads were built at right angles to the penetration roads to produce a fishbone structure that allowed for more access to clear areas for farming. At this point, pasture-forest boundaries developed, resulting in fragmentation of forest habitats. Soon, these state-enabled small farmers organized into associations and highly-capitalized farmers and cattle ranchers began working the lands. The “fishbone” structure of land distribution was replaced by larger blocks of cleared land, needed for heavy machinery to perform better. Private agricultural enterprises began to take over and convert these areas into large plantations.8 Today, the initial drivers of deforestation have been displaced by agricultural enterprises that are linked to global markets. One of these global enterprises is the palm oil industry.
Palm Oil Trees: Benefit or Threat?
Palm oil has become the most traded vegetable oil on the planet. Its high yield, four times as much as that of other oil crops, its versatility, and low cost are a perfect match for the oil industry, accounting for one-third of the vegetable oil produced globally. In the decade between 2003 and 2013, palm oil production doubled and because palm oil trees grow in moist, tropical areas, the Amazon rainforest comprises the largest and most threatened area of vulnerable forest in the South American continent. In other words, this area is the most agriculturally suitable for the plantation of palm oil trees. As is known, this rainforest is species and carbon-rich and the palm oil industry expansion comes at its expense. A study conducted to assess the impacts of the palm oil industry on deforestation and biodiversity loss suggests that the Amazon Rainforest, among other tropical moist forests, is experiencing high rates of deforestation due to the replacement of natural forests with monoculture of palm tree plantations. It also suggests that deforestation increments carbon emissions, reduces plant diversity, and produces permanent loss of many animal species whose lives depend on natural forests.9
Efforts have been made to slow down deforestation from palm oil expansion, but the scenario shows a rapid forest-plantation transition. The organization in charge of certifying palm oil is the Roundtable on Sustainable Palm Oil (RSPO).10 RSPO unites palm oil producers, processors, manufacturers, traders, investors, NGO’s, among others and requires palm oil producers to be aligned with specific criteria to ensure transparency in the management and conservation of resources as well as in the social and environmental assessments of the impacts of palm oil production on the environment. RSPO standards demand that best practices be put in place to reduce deforestation and the conversion of forests into plantation fields. Up to 2016, 3.51 million hectares of RSPO-certified palm oil plantations existed, which produced 13.18 million metric ton of palm oil. Controversies arise because primary and high value forests are regulated and protected, but secondary or regenerating forests are not. Moreover, some palm oil that is sold in the global market comes from uncertified sources. RSPO regulations have been criticized and dubbed as insufficient.11 Nonetheless, RSPO efforts and standards are crucial in the path towards better sustainability in the palm oil industry.
Riverbank Degradation: The Effects of Palm Oil Production on Aquatic and Semi-Aquatic Organisms
Aquatic ecosystems are one of the environments most affected by this industry. The negative impact on these aquatic zones is mainly due to the reduction in native riparian vegetation from the area. Removing vegetation can disrupt aquatic populations as well as cause an increase in temperature, produce changes in the food chain, and increase the levels of soil erosion.12 Because the Amazon waters allowed for longer food chains and more niches, this area has been long characterized by a rich diversification of species, habitat specialization, and formation of assemblages.13 However, recent findings indicate increased pH levels and debris levels in the streams in palm oil plantations are structurally distinct from reference streams in native forest ecosystems. These changes in streams have compromised many species. For example, the abundance of Plecoptera and Trichoptera (PT) assemblages has been noted to be significantly higher in forest streams in contrast to that of palm oil streams, considering that these species need specific conditions in order to have a healthy assemblage. (Fig. 1)
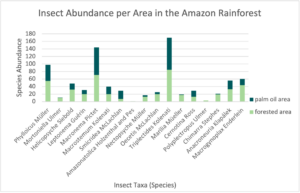
Similarly, aquatic macro-invertebrates such as insects are more common and diverse in forested regions compared to plantation areas. Ephemeroptera (mayfly), like fish, are good biological indicators for these regions. Mayfly assemblies can have a variety of characteristics that can indicate impacts across areas of significant size. The majority of these insects’ lives are spent in streams and lakes, whose waters are affected by precipitation runoff from the region’s drainage basin. This is yet another reason why riparian zones are so important; they act as buffer zones from runoff pesticides and sediments.17 A comparison between mayfly genera showed how they responded to disturbances of their natural habitat and how this affected nymph distribution, composition, and richness. The study compared streams found in natural forest areas and plantation areas. Although the study suggested palm oil plantations contributed to a decrease in diversity and richness, it did not significantly change the mayfly community composition nor mean abundance.18
Into the Trees: The Decline of Functional Diversity in Bird Communities
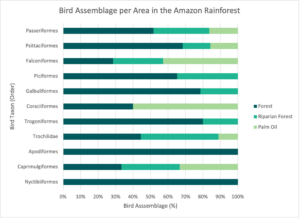
Similar to streams, forested habitats, with their diverse and intricate structures, support a wide array of ecological niches and a diverse local biodiversity, while palm oil plantations offer more uniform environments with less intricate structures, limiting suitable habitats and resources for a diverse range of bird species. Palm oil plantations are considered poor substitutes for native tropical forests because palm trees have a more uniform age, a low canopy cover, scarce foliage, unstable microclimates, and are replaced every 25–30 years.19Palm plantations are also structurally less complex than normal, stratified (layered) tropical forests, and the many species that depend on natural forest habitats are negatively affected by these simpler aspects, posing a serious threat to the preservation of tropical biodiversity.
From a functional diversity point of view, that is, how species use specific characteristics to thrive, the change from forests to palm oil plantations reduces the variety of birds’ functional traits. Bird communities in palm oil plantations exhibit more scattered functional traits, while those in forest habitats display more clustered functional traits. This conversion of forests into palm oil monocultures not only reduces functional diversity and interactions among bird species but results in changes in the functional composition and interactions among bird communities as well. The impact within species of scattered patterns resulting from such transformation is evident in traits such as diet and in the layers in which species look for food, known as foraging strata. Unlike forested habitats that allow for various foraging strategies among different bird species and vertical stratification, palm oil plantations do not supply to species utilizing diverse foraging layers, like the understory layer, found under the canopy. Moreover, resource and shelter availability for coexisting bird species becomes reduced, and the limited types of ecological niches that are available in palm oil plantations contribute even more to this scattering; mainly supporting bird groups with restricted functional characteristics.20
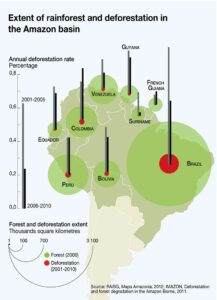
The Amazon rainforest, one of the largest carbon reservoirs on Earth, stores up to 200 PgC in its living biomass and soils, plays a critical role in water, energy, and carbon cycles locally and globally.25 Deforestation also affects climate by altering energy, mass and momentum fluxes between climate subsystem energy reservoirs. These fluxes refer to changes in energy between systems like the atmosphere and the surface where energy is usually stored.26 Through agricultural deforestation, the Amazon rainforest’s original vegetation has been reduced by about 20% in recent decades, leading to changes in rainfall cycles and an increase in periods of extreme drought. (Fig. 3) 27
Deforestation is also associated with increased CO2 and other greenhouse gas emissions, as crop and degraded lands tend to hold less carbon than the forests did before they were cleared. Forest clearing in the Amazon releases all of the carbon that was trapped within the trees and vegetation into the atmosphere. In parallel, the removal of vegetation limits the capacity of carbon storage.28 Research by NASA shows that regional climate is not only affected by the release of greenhouse gasses, but also by shifts in bio- and geo-physical properties that influence local climate by affecting water and energy budgets that govern the balance between energy that reaches Earth from the sun, and energy that flows back from Earth into space.29
In addition, the change from forest to cropland and grassland increases surface the albedo, or reflectivity of the Earth, which cools down surfaces as it reduces the absorption of solar radiation; however, the reduction of forest areas and canopy interception aids evapotranspiration which leads to an increase in surface temperature. Surface temperature increases due to more solar radiation reaching the ground. Besides an increase in temperature, the loss of vegetation directly affects the hydrological cycle as evaporation and transpiration from vegetation decreases.30
As cropland density appears to be one of the main drivers for deforestation, spillover effects from agriculture affect the rainforest indirectly. Through different time periods significant differences in the amazon biome occur as the dynamics of forest cover is impacted by a diversity in deforestation. When compared to non deforested areas that absorb carbon over time thanks to net primary productivity, deforested areas are warmer and drier and their soil carbon net gain is higher. 3132
The Tipping Point: From Palm Oil Deforestation to Climate Crisis
Data from 2010 in a study by Brazilian Cerrado Conservation predict that, in this century, the temperature in this area will increase by 3o degrees Centigrade, and the amount of precipitation will decrease by 20 percent.33 These drastic changes caused by deforestation and/or climate change may cause the Amazon rainforest to reach its threshold, cross an ecological tipping point, and suffer a “radical restructuring and re-sorting of the forest community”.34
A tipping point in an ecological system refers to the critical moment when it only takes a small additional disturbance considerably to affect its characteristics and modifies its “ state or development”. For the Amazon Rainforest, scientists claim that its tipping point, if crossed, would extend its dry season and the rainforest would experience a drier equilibrium, one which would transform the rainforest into a savanna or seasonal forest. Such prediction is backed up by evidence from paleoecology and ecophysiology which revealed that some 8,000 to 3,600 years ago harsh ecosystem transformations had occurred due to climate change and reduction of the rainforest.35
Paving the Path Towards Sustainability
The efficiency of palm oil is undeniable, but unfortunately “deforestation-free” palm oil does not exist. According to FAO data, the percent increase in total planted areas from 1989 to 2013 for this crop in Brazil alone was 77%. An assessment about oil palm vulnerable forests that was conducted using the FAO’s Global Agro-Ecological Zones (GAEZ) model for agricultural suitability of palm oil, showed that in South America, up to the year 2016, 4,418,443 km 2 were still vulnerable forests and only 9.4% were protected forests.36
Expanding this industry at the expense of losing even more natural forests is not an option.37 Neither is shifting the oil industry to other oils with a lower yield. This would cause more use of land somewhere else, and the oil industry would face the same environmental issues. Moreover, many local and small farmers still depend on their production of palm oil for their livelihoods.
A legacy of lost forests calls for global and local measures; it is a concern that must be addressed by all parties.including government regulations and law enforcements, environmental monitoring and interventions, and voluntary market initiatives from buyers and sellers. RSPO must propose new and improved sustainable harvesting techniques, continuously revise them, and guarantee their implementation by all palm oil producers.38 Moreover, strategies such as the identification of rare or endangered animal species that are unlikely to adapt to a continued habitat and climate change to ensure their long-term survival, the modification of the industry’s supply chain, and the creation of international climate agreements must be put in place.39
Conclusions
In recent years, especially after 2013, the growing demand for vegetable oil has catapulted the expansion of palm oil plantations in the Amazon. As the industry grows deeper into the forest, it not only degrades and changes the landscape, but it also endangers the most biologically diverse biome on the planet. 40 The amount of life that the Amazon sustains is copious, and consequences for the loss of its biodiversity are severe and complex, ranging from environmental to socioeconomic effects.
The system began its shift mainly due to the supply and demand for global market products such as biodiesel, cooking oil, packed and fast food, personal care products, cosmetics, household cleaners, among others and that could be manufactured from palm oil.41 Since then, small-scale agriculture in the area was replaced by large operators and manufacturers, and transformation in the land use inevitably altered the balance in the ecosystem’s biodiversity and climate dynamics.42
Data from a 20+ year study of landscape change due to the expansion of the palm oil industry in the Brazilian Amazon exhorts the creation of appropriate land use policies that focus on landscape integrity and the development of strategies and initiatives that help regulate monocultures in large areas in order to safeguard and guarantee the rainforest’s sustainability and biodiversity. The data also demonstrated a striking increase in deforestation of the region, where forest loss accounted for almost 50%. 43 This finding resonates with the notion that the degradation and fragmentation of the land due to palm oil cultivations will continue to reduce the Amazon Forest and that there is an urgent need for actions that will guarantee the protection and proportions of forests amidst the palm oil plantations.44
Stakeholders, government officials, environmentalists, non-governmental organization representatives, climatologists and all other humans that are directly or indirectly involved in the palm oil industry must acknowledge that the Amazon rainforest is a huge ecological treasure. That its preservation will not only benefit the oil market they advocate for, but that it is essential for maintaining the delicate balance between plant and animal species, for keeping greenhouse gasses at check, and for giving our planet abundant oxygen to sustain life. The choice is simple yet determinant. Will humans be agents of destruction or agents of restoration? The future of the Amazon rainforest, and even perhaps existence, relies on the answer.
- Vijay, Varsha, Stuart L. Pimm, Clinton N. Jenkins, and Sharon J. Smith. 2016. “The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss.” Edited by Madhur Anand. PLOS ONE 11 (7): e0159668. https://doi.org/10.1371/journal.pone.0159668. ↵
- Malhado, Ana Cláudia Mendes, Gabrielle Ferreira Pires, and Marcos Heil Costa. 2010. “Cerrado Conservation Is Essential to Protect the Amazon Rainforest.” AMBIO 39 (8): 580–84. https://doi.org/10.1007/s13280-010-0084-6. ↵
- Malhado, Ana Cláudia Mendes, Gabrielle Ferreira Pires, and Marcos Heil Costa. 2010. “Cerrado Conservation Is Essential to Protect the Amazon Rainforest.” AMBIO 39 (8): 580–84. https://doi.org/10.1007/s13280-010-0084-6. ↵
- Rudel, Thomas K., Ruth Defries, Gregory P. Asner, and William F. Laurance. 2009. “Changing Drivers of Deforestation and New Opportunities for Conservation.” Conservation Biology 23 (6): 1396–1405. https://doi.org/10.1111/j.1523-1739.2009.01332.x. ↵
- Rudel, Thomas K., Ruth Defries, Gregory P. Asner, and William F. Laurance. 2009. “Changing Drivers of Deforestation and New Opportunities for Conservation.” Conservation Biology 23 (6): 1396–1405. https://doi.org/10.1111/j.1523-1739.2009.01332.x. ↵
- Rudel, Thomas K., Ruth Defries, Gregory P. Asner, and William F. Laurance. 2009. “Changing Drivers of Deforestation and New Opportunities for Conservation.” Conservation Biology 23 (6): 1396–1405. https://doi.org/10.1111/j.1523-1739.2009.01332.x. ↵
- Rudel, Thomas K., Ruth Defries, Gregory P. Asner, and William F. Laurance. 2009. “Changing Drivers of Deforestation and New Opportunities for Conservation.” Conservation Biology 23 (6): 1396–1405. https://doi.org/10.1111/j.1523-1739.2009.01332.x. ↵
- Rudel, Thomas K., Ruth Defries, Gregory P. Asner, and William F. Laurance. 2009. “Changing Drivers of Deforestation and New Opportunities for Conservation.” Conservation Biology 23 (6): 1396–1405. https://doi.org/10.1111/j.1523-1739.2009.01332.x. ↵
- Vijay, Varsha, Stuart L. Pimm, Clinton N. Jenkins, and Sharon J. Smith. 2016. “The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss.” Edited by Madhur Anand. PLOS ONE 11 (7): e0159668. https://doi.org/10.1371/journal.pone.0159668. ↵
- Vijay, Varsha, Stuart L. Pimm, Clinton N. Jenkins, and Sharon J. Smith. 2016. “The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss.” Edited by Madhur Anand. PLOS ONE 11 (7): e0159668. https://doi.org/10.1371/journal.pone.0159668. ↵
- Vijay, Varsha, Stuart L. Pimm, Clinton N. Jenkins, and Sharon J. Smith. 2016. “The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss.” Edited by Madhur Anand. PLOS ONE 11 (7): e0159668. https://doi.org/10.1371/journal.pone.0159668. ↵
- Ferreira, Márcio Cunha, Tiago Octavio Begot, Bruno Da Silveira Prudente, Leandro Juen, and Luciano Fogaça De Assis Montag. 2018. “Effects of Oil Palm Plantations on Habitat Structure and Fish Assemblages in Amazon Streams.” Environmental Biology of Fishes 101 (4): 547–62. https://doi.org/10.1007/s10641-018-0716-4. ↵
- Ferreira, Márcio Cunha, Tiago Octavio Begot, Bruno Da Silveira Prudente, Leandro Juen, and Luciano Fogaça De Assis Montag. 2018. “Effects of Oil Palm Plantations on Habitat Structure and Fish Assemblages in Amazon Streams.” Environmental Biology of Fishes 101 (4): 547–62. https://doi.org/10.1007/s10641-018-0716-4. ↵
- De Paiva, Carina Kaory Sasahara, Ana Paula Justino De Faria, Lenize Batista Calvão, and Leandro Juen. 2017. “Effect of Oil Palm on the Plecoptera and Trichoptera (Insecta) Assemblages in Streams of Eastern Amazon.” Environmental Monitoring and Assessment 189 (8): 393. https://doi.org/10.1007/s10661-017-6116-y. ↵
- Ferreira, Márcio Cunha, Tiago Octavio Begot, Bruno Da Silveira Prudente, Leandro Juen, and Luciano Fogaça De Assis Montag. 2018. “Effects of Oil Palm Plantations on Habitat Structure and Fish Assemblages in Amazon Streams.” Environmental Biology of Fishes 101 (4): 547–62. https://doi.org/10.1007/s10641-018-0716-4. ↵
- Ferreira, Márcio Cunha, Tiago Octavio Begot, Bruno Da Silveira Prudente, Leandro Juen, and Luciano Fogaça De Assis Montag. 2018. “Effects of Oil Palm Plantations on Habitat Structure and Fish Assemblages in Amazon Streams.” Environmental Biology of Fishes 101 (4): 547–62. https://doi.org/10.1007/s10641-018-0716-4. ↵
- Shimano, Yulie, and Leandro Juen. 2016. “How Oil Palm Cultivation Is Affecting Mayfly Assemblages in Amazon Streams.” Annales de Limnologie – International Journal of Limnology 52: 35–45. https://doi.org/10.1051/limn/2016004. ↵
- Shimano, Yulie, and Leandro Juen. 2016. “How Oil Palm Cultivation Is Affecting Mayfly Assemblages in Amazon Streams.” Annales de Limnologie – International Journal of Limnology 52: 35–45. https://doi.org/10.1051/limn/2016004. ↵
- Almeida, Sara M., Larissa C. Silva, Maíra R. Cardoso, Pablo V. Cerqueira, Leandro Juen, and Marcos P. D. Santos. 2016. “The Effects of Oil Palm Plantations on the Functional Diversity of Amazonian Birds.” Journal of Tropical Ecology 32 (6): 510–25. https://doi.org/10.1017/S0266467416000377. ↵
- Almeida, Sara M., Larissa C. Silva, Maíra R. Cardoso, Pablo V. Cerqueira, Leandro Juen, and Marcos P. D. Santos. 2016. “The Effects of Oil Palm Plantations on the Functional Diversity of Amazonian Birds.” Journal of Tropical Ecology 32 (6): 510–25. https://doi.org/10.1017/S0266467416000377. ↵
- Almeida, Sara M., Larissa C. Silva, Maíra R. Cardoso, Pablo V. Cerqueira, Leandro Juen, and Marcos P. D. Santos. 2016. “The Effects of Oil Palm Plantations on the Functional Diversity of Amazonian Birds.” Journal of Tropical Ecology 32 (6): 510–25. https://doi.org/10.1017/S0266467416000377. ↵
- Almeida, Sara M., Larissa C. Silva, Maíra R. Cardoso, Pablo V. Cerqueira, Leandro Juen, and Marcos P. D. Santos. 2016. “The Effects of Oil Palm Plantations on the Functional Diversity of Amazonian Birds.” Journal of Tropical Ecology 32 (6): 510–25. https://doi.org/10.1017/S0266467416000377. ↵
- Almeida, Sara M., Larissa C. Silva, Maíra R. Cardoso, Pablo V. Cerqueira, Leandro Juen, and Marcos P. D. Santos. 2016. “The Effects of Oil Palm Plantations on the Functional Diversity of Amazonian Birds.” Journal of Tropical Ecology 32 (6): 510–25. https://doi.org/10.1017/S0266467416000377. ↵
- https://www.flickr.com/photos/gridarendal/31519187894 ↵
- Jiang, Yelin, Guiling Wang, Weiguang Liu, Amir Erfanian, Qing Peng, and Rong Fu. 2021. “Modeled Response of South American Climate to Three Decades of Deforestation.” Journal of Climate 34 (6): 2189–2203. https://doi.org/10.1175/JCLI-D-20-0380.1. ↵
- Longobardi, Patrick, Alvaro Montenegro, Hugo Beltrami, and Michael Eby. 2016. “Deforestation Induced Climate Change: Effects of Spatial Scale.” Edited by Juan A. Añel. PLOS ONE 11 (4): e0153357. https://doi.org/10.1371/journal.pone.0153357. ↵
- Santos, Gustavo André de Araújo, Luiz Fernando Favacho Morais Filho, Kamila Cunha de Meneses, Carlos Antonio da Silva Junior, Glauco de Souza Rolim, and Newton La Scala. 2022. “Hot Spots and Anomalies of CO2 over Eastern Amazonia, Brazil: A Time Series from 2015 to 2018.” Environmental Research 215 (December): 114379. https://doi.org/10.1016/j.envres.2022.114379. ↵
- Longobardi, Patrick, Alvaro Montenegro, Hugo Beltrami, and Michael Eby. 2016. “Deforestation Induced Climate Change: Effects of Spatial Scale.” Edited by Juan A. Añel. PLOS ONE 11 (4): e0153357. https://doi.org/10.1371/journal.pone.0153357. ↵
- What is Earth’s Energy Budget? Five Questions with a Guy Who Knows – NASA. 2017 Apr 10. https://www.nasa.gov/centers-and-facilities/langley/what-is-earths-energy-budget-five-questions-with-a-guy-who-knows/. ↵
- Jiang, Yelin, Guiling Wang, Weiguang Liu, Amir Erfanian, Qing Peng, and Rong Fu. 2021. “Modeled Response of South American Climate to Three Decades of Deforestation.” Journal of Climate 34 (6): 2189–2203. https://doi.org/10.1175/JCLI-D-20-0380.1. ↵
- Kuschnig, Nikolas, Jesús Crespo Cuaresma, Tamás Krisztin, and Stefan Giljum. 2021. “Spatial Spillover Effects from Agriculture Drive Deforestation in Mato Grosso, Brazil.” Scientific Reports 11 (1): 21804. https://doi.org/10.1038/s41598-021-00861-y. ↵
- Longobardi, Patrick, Alvaro Montenegro, Hugo Beltrami, and Michael Eby. 2016. “Deforestation Induced Climate Change: Effects of Spatial Scale.” Edited by Juan A. Añel. PLOS ONE 11 (4): e0153357. https://doi.org/10.1371/journal.pone.0153357. ↵
- Malhado, Ana Cláudia Mendes, Gabrielle Ferreira Pires, and Marcos Heil Costa. 2010. “Cerrado Conservation Is Essential to Protect the Amazon Rainforest.” AMBIO 39 (8): 580–84. https://doi.org/10.1007/s13280-010-0084-6. ↵
- Malhado, Ana Cláudia Mendes, Gabrielle Ferreira Pires, and Marcos Heil Costa. 2010. “Cerrado Conservation Is Essential to Protect the Amazon Rainforest.” AMBIO 39 (8): 580–84. https://doi.org/10.1007/s13280-010-0084-6. ↵
- Malhado, Ana Cláudia Mendes, Gabrielle Ferreira Pires, and Marcos Heil Costa. 2010. “Cerrado Conservation Is Essential to Protect the Amazon Rainforest.” AMBIO 39 (8): 580–84. https://doi.org/10.1007/s13280-010-0084-6. ↵
- Vijay, Varsha, Stuart L. Pimm, Clinton N. Jenkins, and Sharon J. Smith. 2016. “The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss.” Edited by Madhur Anand. PLOS ONE 11 (7): e0159668. https://doi.org/10.1371/journal.pone.0159668. ↵
- Vijay, Varsha, Stuart L. Pimm, Clinton N. Jenkins, and Sharon J. Smith. 2016. “The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss.” Edited by Madhur Anand. PLOS ONE 11 (7): e0159668. https://doi.org/10.1371/journal.pone.0159668. ↵
- Vijay, Varsha, Stuart L. Pimm, Clinton N. Jenkins, and Sharon J. Smith. 2016. “The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss.” Edited by Madhur Anand. PLOS ONE 11 (7): e0159668. https://doi.org/10.1371/journal.pone.0159668. ↵
- Vijay, Varsha, Stuart L. Pimm, Clinton N. Jenkins, and Sharon J. Smith. 2016. “The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss.” Edited by Madhur Anand. PLOS ONE 11 (7): e0159668. https://doi.org/10.1371/journal.pone.0159668. ↵
- De Almeida, Arlete Silva, Ima Célia Guimarães Vieira, and Silvio F.B. Ferraz. 2020. “Long-Term Assessment of Oil Palm Expansion and Landscape Change in the Eastern Brazilian Amazon.” Land Use Policy 90 (January): 104321. https://doi.org/10.1016/j.landusepol.2019.104321. ↵
- Vijay, Varsha, Stuart L. Pimm, Clinton N. Jenkins, and Sharon J. Smith. 2016. “The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss.” Edited by Madhur Anand. PLOS ONE 11 (7): e0159668. https://doi.org/10.1371/journal.pone.0159668. ↵
- Vijay, Varsha, Stuart L. Pimm, Clinton N. Jenkins, and Sharon J. Smith. 2016. “The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss.” Edited by Madhur Anand. PLOS ONE 11 (7): e0159668. https://doi.org/10.1371/journal.pone.0159668. ↵
- De Almeida, Arlete Silva, Ima Célia Guimarães Vieira, and Silvio F.B. Ferraz. 2020. “Long-Term Assessment of Oil Palm Expansion and Landscape Change in the Eastern Brazilian Amazon.” Land Use Policy 90 (January): 104321. https://doi.org/10.1016/j.landusepol.2019.104321. ↵
- Laurance, William F., Heraldo L. Vasconcelos, and Thomas E. Lovejoy. 2000. “Forest Loss and Fragmentation in the Amazon: Implications for Wildlife Conservation.” Oryx 34 (1): 39–45. https://doi.org/10.1046/j.1365-3008.2000.00094.x. ↵



