
In September of 1928, at St. Mary’s Hospital in London, a profound day in medical history occurred when Alexander Fleming noticed something peculiar on his Petri dish: a mold. Alexander stood silently in front of the Petri dish with mold streaks running across its translucent surface. With his eyes narrowed in focus, he leaned in, and in an instant, Fleming yearned to unravel the scientific mystery behind this accidental phenomenon. He had no idea that he was standing on the brink of history at that inconspicuous moment. It was in the calm of his laboratory that Alexander Fleming would make a discovery that would pave the way for one of the greatest medical breakthroughs in history.1
In the nineteenth century, the inability to effectively treat minor infections and wounds resulted in numerous deaths. In actuality, during this period, infectious diseases were the leading cause of death.2 Many doctors desperately hoped that there would be a treatment for the infections that were killing so many people. This continued into the twentieth century, a time marked by many medical challenges related to bacterial infections.

In order to understand the nature of disease and infection, the concept of cells must be understood. In fact, every living thing—plant, animal, and bacterium—is made of cells. Plant and animal cells are eukaryotes, meaning they have a nucleus and membrane-bound organelles, whereas bacteria are single-celled prokaryotic organisms and do not have a nucleus. While not all bacteria pose immediate threats, many harbor the potential to cause infections and diseases, some of which can be fatal. Diseases occur via infections either from bacteria or viruses. Our bodies try to protect us by creating antibodies to attack and destroy pathogens; however, sometimes that is not enough. In these scenarios, pathogens take over and cause infection to the human body.3
Alexander Fleming, a recent medical school graduate, joined the British Royal Army Medical Corps in 1914 at the beginning of World War I and worked as a bacteriologist. During this time, he was researching at a laboratory in Boulogne to find a cure for bacterial infections.4 Fleming oversaw identifying the bacteria on wounded soldiers by taking swabs and irrigating the wounds with antiseptics. He cultured the bacteria from the swabs and identified the most prevalent bacteria found in the wounds.5 Additionally, he noticed that the antiseptics were doing more damage and not killing the bacteria, which demonstrated that using antiseptics was somehow ineffective. Fleming was unsure how to combat these diseases but knew antiseptics were not the answer. In 1918, he returned to St. Mary’s in London to continue working on finding a chemical cure for infection.6
The horrors of World War I had a lasting impact on Fleming as he witnessed firsthand the tragic effects of bacterial infections that led to the deaths of countless soldiers. Although many soldiers died from combat, infections from wounds were leading to sepsis and death. The early 1900s was “plagued with people dying from many sicknesses and diseases.”7 Many medical challenges were imposed by bacterial infections during the twentieth century. For example, people were dying of various sicknesses and diseases like strep throat, scarlet fever, gonorrhea, and meningitis, all of which could be easily cured with antibiotics.8 During this time, people were wondering if they were going to live to see the next day, as even something as trivial as a common cold could lead to death.

In 1921, Fleming endured a cold and analyzed his own nasal mucus. He discovered a new bacteriolytic agent known as a lysozyme—an antibacterial enzyme found in many bodily fluids such as tears, saliva, and mucus.9 Collaborating with other scientists, Fleming established that lysozymes were normal biological components of various bodily fluids. They are biological protective forces that our bodies use to kill bacteria. Although this discovery had little therapeutic implications, it was an important discovery; however, it received little recognition from other scientists. Nonetheless, Fleming recognized his own achievement and continued moving forward with his research.10
In the crisp August of 1928, amidst the bustling laboratories of St. Mary’s Hospital in London, Dr. Alexander Fleming was meticulously arranging Petri dishes upon his cluttered desk. With precise movements, he was preparing his cultures, each containing a colony of staphylococci, a gram-positive bacterium, which has a specific type of cell wall.11 Despite the impending departure for his well-deserved vacation, his focus remained unwavering as he moved the Petri dishes by stacking them in a bath of antiseptic. Oblivious to the impending twist of destiny, Fleming entrusted the care of his cultures to a bath of antiseptic; however, he did not realize there was not enough antiseptic to cover all the plates. He unknowingly set the stage for a medical revelation that would change the world forever.12 Weeks passed, and it was not until September 3, when he returned to the familiarity of his laboratory, that the significance of his oversight would come to light. Disappointment swept over him as he examined the remains of his experiment and discarded many of the Petri dishes. But amidst the discarded remnants of his failed endeavors, something caught his eye. Beneath the sea of abandoned Petri dishes, he noticed something unique on some of the dishes: mold. In that moment, as curiosity ignited, Fleming knew he discovered something special that would alter the course of modern medicine.13
Fleming examined the mold at St. Mary’s Hospital in London and quickly realized he was making a significant medical breakthrough. Staphylococcal colonies had “become translucent” and were dying around the mold, which was an extraordinary phenomenon that demanded investigation.14 Since the original mold was in pure culture, Fleming transferred it to a new culture plate and, after allowing it to grow for four or five days, he streaked different microbes radially across the surface. While some grew across the molds, others were inhibited. Based on this, Fleming concluded that the antibacterial substance secreted by the mold had inhibitory effects on a very exceptional kind of bacteria.15
Fleming then grew the mold on a fluid medium, and repeated the testing process he used for the lysozyme to see if an antiseptic substance appeared in the fluid. He positioned it within a groove on a culture plate and spread a variety of microbes across it. Subsequently, he noted that the microbes most inhibited belonged to the group of bacteria most frequently associated with infections. Fleming also tested the sensitivity of the different microbes, discovering that some were unaffected while many were inhibited. The mold also possessed the crucial characteristic of being extraordinarily strong. The mold could prevent staphylococci from growing even if the culture fluid was diluted a thousand times, proving the specificity of this. Thus, in addition to killing the bacteria, this mold also had inhibitory effects.16
As Fleming delved deeper into his investigation of the mysterious mold, a sense of exhilaration coursed through his veins. His excitement increased with every new finding and insight he gained, fueled by the realization of the enormous implications this mold held. Through continued experiments, Fleming noticed that it caused lytic changes in the bacteria, indicating that it could diffuse freely—an essential characteristic for an antibacterial agent used internally in humans. Using his own blood, Fleming discovered that while it could stop bacteria from growing, it had no toxic effects on humans. Also, additional experiments showed there was no toxicity in animals either. “It was the first substance I had ever tested which was more antibacterial than it was antileukocytic,” Fleming said, suggesting that it could be used therapeutically to treat infections at the right concentrations and with the right handling. Each revelation was a triumph, a testament to his unwavering dedication and the limitless possibilities of scientific inquiry.17 Though Fleming faced challenges due to it being very unstable and challenging to work with, along with the limitations of his expertise as a bacteriologist rather than a chemist, he remained undeterred. He envisioned a future where his miraculous mold could be concentrated and purified to combat the scourge of infectious diseases. Here, at the St. Mary’s Hospital in London, he stood on the brink of greatness, aware of the potential this mold held. Fleming recognized that the journey ahead would be difficult and uncertain; however, his heart raced as he grasped the profound significance of the knowledge he possessed—the key to revolutionizing medicine. With his groundbreaking discovery, there was boundless potential and a promise of a brighter future.18
At the beginning of March 1929, Fleming understood the significance of his findings and tagged this active ingredient in the mold as Penicillium notatum (Penicillin).19 Penicillin belongs to the B-lactam class of antibiotics, known for their ability to target and disrupt the bacterial cell wall, ultimately leading to bacterial cell death. He published his findings in the British Journal of Experimental Pathology later that year, and on February 13, 1929, he gave a presentation of his findings at the Medical Research Club. Until 1936, he mentioned penicillin in one or two publications, but many ignored it until years later. His co-scientists Dr. Ernst Chain and Sir Howard Florey used the strain of Penicillium notatum and succeeded in concentrating it. In 1945, Fleming and his colleagues shared a Nobel Prize.20
For Fleming, this was not the end of the story—perhaps only the beginning—as penicillin had the ability to kill many harmful bacteria as never before. Upon discovery, penicillin was difficult to work with due to instability; however, other chemists collaborated with Fleming and identified the scientific structure to help isolate and synthetically produce penicillin for mass production.21
These findings provided security for individuals with bacterial infections as they were no longer troublesome. Penicillin was the first therapeutic agent to destroy bacteria in vivo and capable of saving millions of lives. Fleming’s discovery led scientists like Howard Florey, Ernst Chain, Norman Heatley, and Edward Abraham to collaborate and prepare a stable form of penicillin. Florey was tasked with looking at the biological elements, while Chain would study the biochemical properties of the mold. The first task was to create enough penicillin to conduct experiments for clinical trials. During this time, these scientists realized that in order to make substantial amounts of penicillin needed for clinical trials they would have to come to the United States due to the lack of resources at the Oxford laboratory. Although production began slowly, with the help of United States government funding, they began to produce penicillin in large quantities. The transition from laboratory penicillin production to industrial production required the help of many scientists, companies, and government agencies.22
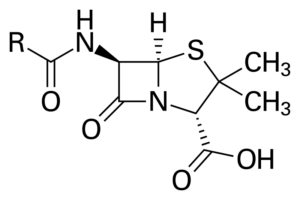
When penicillin finally became mass-produced and used therapeutically, thousands of lives were saved on and off the battlefield. Individuals affected by World War II were being cured using this “miracle drug.”23 It has been estimated that one-third of deaths at the end of the nineteenth century were caused by infectious diseases, but by the end of the twentieth century, the death rate dropped to around 4%.24 This discovery was one of the most powerful achievements that helped trigger the age of medical advancements, specifically in antibiotics.25 The clinical application of antibiotics has revolutionized public health, leading to a dramatic decrease in mortality rates from infections. Penicillin’s unparalleled impact on mortality rates and lifespan extension remains unmatched by any other drug in history, leaving an indelible mark on medical history.26
- J. W. Bennett and Geoffrey M. Gadd, Alexander Fleming and the Discovery of Penicillin, Advances in Applied Microbiology, v. 49 (New York: Academic Press, 2001), 168. ↵
- Nelson Kardos and Arnold L. Demain, “Penicillin: The Medicine with the Greatest Impact on Therapeutic Outcomes,” Applied Microbiology and Biotechnology 92, no. 4 (November 1, 2011), 679. ↵
- Guy De la Bédoyère, The Discovery of Penicillin (Evans Brothers, 2005), 4-13. ↵
- Kevin Brown, “Alexander Fleming | Biography, Education, Discovery, Nobel Prize, & Facts,” Britannica (online), accessed January 22, 2024, https://www.britannica.com/biography/Alexander-Fleming. ↵
- J. W. Bennett and Geoffrey M. Gadd, Alexander Fleming and the Discovery of Penicillin, Advances in Applied Microbiology, v. 49 (New York: Academic Press, 2001), 165-66. ↵
- Stacie L. Derderian, “Alexander Fleming’s Miraculous Discovery of Penicillin,” Rivier Academic Journal 3 No. 2 (2007), 1. ↵
- Stacie L. Derderian, “Alexander Fleming’s Miraculous Discovery of Penicillin,” Rivier Academic Journal 3 No. 2 (2007), 1-2. ↵
- Stacie L. Derderian, “Alexander Fleming’s Miraculous Discovery of Penicillin,” Rivier Academic Journal 3 No. 2 (2007), 2. ↵
- J. W. Bennett and Geoffrey M. Gadd, Alexander Fleming and the Discovery of Penicillin, Advances in Applied Microbiology, v. 49 (New York: Academic Press, 2001), 167. ↵
- J. W. Bennett and Geoffrey M. Gadd, Alexander Fleming and the Discovery of Penicillin, Advances in Applied Microbiology, v. 49 (New York: Academic Press, 2001), 167-68. ↵
- Stacie L. Derderian, “Alexander Fleming’s Miraculous Discovery of Penicillin,” Rivier Academic Journal 3 No. 2 (2007), 2. ↵
- The Discovery of Penicillin, accessed January 22, 2024, https://www.pbslearningmedia.org/resource/odys08.sci.life.gen.discovery/the-discovery-of-penicillin/. ↵
- Alexander Fleming, “Nobel Lecture: Penicillin,” presented at the Nobel Prize Award Ceremony, Stockholm, Sweden, December 11, 1945, https://www.nobelprize.org/uploads/2018/06/fleming-lecture.pdf ↵
- Alexander Fleming, “Nobel Lecture: Penicillin,” presented at the Nobel Prize Award Ceremony, Stockholm, Sweden, December 11, 1945, https://www.nobelprize.org/uploads/2018/06/fleming-lecture.pdf ↵
- Alexander Fleming, “Nobel Lecture: Penicillin,” presented at the Nobel Prize Award Ceremony, Stockholm, Sweden, December 11, 1945, https://www.nobelprize.org/uploads/2018/06/fleming-lecture.pdf ↵
- Alexander Fleming, “Nobel Lecture: Penicillin,” presented at the Nobel Prize Award Ceremony, Stockholm, Sweden, December 11, 1945, https://www.nobelprize.org/uploads/2018/06/fleming-lecture.pdf ↵
- Alexander Fleming, “Nobel Lecture: Penicillin,” presented at the Nobel Prize Award Ceremony, Stockholm, Sweden, December 11, 1945, https://www.nobelprize.org/uploads/2018/06/fleming-lecture.pdf ↵
- Alexander Fleming, “Nobel Lecture: Penicillin,” presented at the Nobel Prize Award Ceremony, Stockholm, Sweden, December 11, 1945, https://www.nobelprize.org/uploads/2018/06/fleming-lecture.pdf ↵
- Stacie L. Derderian, “Alexander Fleming’s Miraculous Discovery of Penicillin,” Rivier Academic Journal 3 No. 2 (2007), 2. ↵
- Alexander Fleming, “Nobel Lecture: Penicillin,” presented at the Nobel Prize Award Ceremony, Stockholm, Sweden, December 11, 1945, https://www.nobelprize.org/uploads/2018/06/fleming-lecture.pdf ↵
- Nelson Kardos and Arnold L. Demain, “Penicillin: The Medicine with the Greatest Impact on Therapeutic Outcomes,” Applied Microbiology and Biotechnology 92, no. 4 (November 1, 2011), 679-80. ↵
- Robert Gaynes, “The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use,” Emerging Infectious Diseases 23, no. 5 (May 2017), 849–53. ↵
- J. W. Bennett and Geoffrey M. Gadd, Alexander Fleming and the Discovery of Penicillin, Advances in Applied Microbiology, v. 49 (New York: Academic Press, 2001), 163. ↵
- Nelson Kardos and Arnold L. Demain, “Penicillin: The Medicine with the Greatest Impact on Therapeutic Outcomes,” Applied Microbiology and Biotechnology 92, no. 4 (November 1, 2011), 678. ↵
- Stacie L. Derderian, “Alexander Fleming’s Miraculous Discovery of Penicillin,” Rivier Academic Journal 3 No. 2 (2007), 4 ↵
- Nelson Kardos and Arnold L. Demain, “Penicillin: The Medicine with the Greatest Impact on Therapeutic Outcomes,” Applied Microbiology and Biotechnology 92, no. 4 (November 1, 2011), 680. ↵



17 comments
Jordan Robbins
Hi, through your writing I learned so much more about Penicillin! Its so crazy how much thought goes into these experiments and also the sacrifices along the way. I appreciate the way you explained his story and his contribution to sciences! Also the infographic looks great!
Mariana Chamorro
Callah, your article on Alexander Fleming’s discovery of penicillin is awesome! I love how you described the moment he noticed the mold on his Petri dish and the excitement he must have felt. Your explanation of how penicillin was developed from that discovery to become a lifesaving drug is really interesting. Great job!
Carlos Anthony Alonzo
Congratulations on your research! Your study on Alexander Fleming’s discovery of penicillin is nice article on the field of medicine, offering valuable insights into how medicine is made. Your methodology is great, and your sources are well-supported by the evidence. I appreciate the clarity of your presentation and the depth of your analysis. I look forward to seeing how your articles and research continues to evolve at St. Mary’s University!
Lauren Sahadi
I’ve always known about penicillin, but never where it came from. It’s interesting to know that something that big could result from an accident that involved mold. Through innovation, penicillin became a lifesaving treatment that has helped a lot of people. This article shows how curiosity can lead to great things. Really great job telling the story of how a well known drug in the medical industry was accidentally discovered.
Martin Martinez
I did additional research, and it’s a bit sad that people in the United States tried to patent the method of producing penicillin when Fleming offered it to the world for free. We see this with Tesla’s inventions, and there are likely other helpful resources which have only been seen as profitable. But that does not reduce the impact that penicillin left on the world of medicine.
Fernando Milian
Alexander Fleming’s discovery of penicillin is incredible! It’s like a dramatic movie scene where he comes back from vacation and finds this unexpected mold that turned out to be a massive breakthrough in medicine. It’s amazing to think that just by observing that mold, he started the whole journey of antibiotics, which has saved millions of lives since then. The part about him testing different bacteria against the mold and seeing how they reacted differently really shows how careful and detailed his experiments were. It’s inspiring to see how one person’s curiosity and determination can lead to such a huge impact on the world.
Sebastian Hernandez-Soihit
Amazing portrayal of Fleming’s journey and the collaborative efforts that followed to harness the power of this groundbreaking antibiotic. It effectively highlights the profound impact of penicillin on public health and its role in bringing forth a new era of medical advancements. Does a good job in honoring the legacy of Fleming
Nicholas Pigott
Hi Callah! Your article is a fantastic description of one of the greatest human achievements of all time, and is a true testament to the power of mankind and science. You hit all the right notes in this article, and successfully conveyed all the information. I also loved all the photographs included in the article! Antibiotics like penicillin is a luxury that we now take for granted too often. Congratulations on your nomination!
Joseph Reed
I think it would do us well to remember how dangerous bacteria and other sicknesses were to humans up until only a century ago; it has been less than 100 years since penicillin was discovered by Dr. Fleming, and since then, his discovery paved the way for a safer, healthier world. I doubt many of us would even be here today were it not for his invention of penicillin and antibiotics, which have saved previous generations from one of the scourges of our planet, disease.
Esmeralda Gomez
Reading this article on Alexander Fleming, the pioneer behind penicillin, was unexpectedly intriguing. Exploring the scientific journey and the profound impact of his breakthrough was quite a read. I had read of the history of penicillin before, but never dipped too much into the history, this article serves as a great recap of this topic and provides an enriching experience. Kudos to the writer for their insightful work and congratulations on the nomination!
Looking forward to seeing more scholarly yet engaging reads from you!