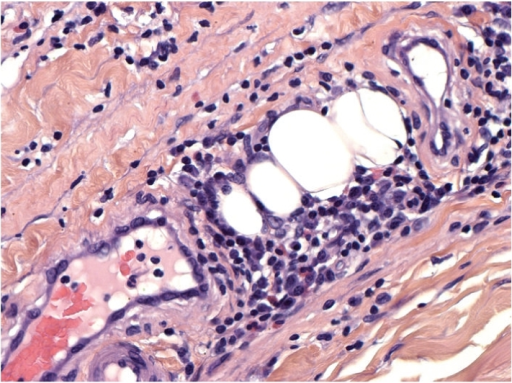
What do the flu, cold, salmonella, malaria, pneumonia, and other diseases like meningitis share in common? These are all well-studied diseases whose causes and workings have been identified by the scientific community. Thanks to studies which have identified these properties of the diseases, it has been possible to develop preventative measures and treatments for each. The same can’t be said for lesser-known diseases. Consider the disease eosinophilic fasciitis. Like most, it’s likely this disease sounds unfamiliar and foreign given that there have been less than 300 reported cases.1 As a result, there is a void of information regarding how this disease develops. In addition, this lack of research about its development can contribute to incorrect diagnoses. An improper diagnosis can result in improper treatment, and the disease can continue to exist for months or even years before it is properly identified. In order to understand why there is a lack of information, the results of which could lead to an incorrect diagnosis, it’s important to understand how this disease was first discovered.
Officially named in 1975, eosinophilic fasciitis is given its disease name following an initial analysis of the properties it exhibits. Its name was derived following the initial observation of peripheral eosinophilia in the form of eosinophilic infiltration (eosinophilic-) and inflammation of the fascia (-fasciitis).2 For additional context, peripheral eosinophilia refers to the increased number of eosinophils in the peripheral blood – specifically having more than 500 eosinophils per microliter of blood. Eosinophils are a type of white blood cell and is known to have a role in regulating local inflammatory responses through secreted proteins like IL-5 and IL-6.3 The fascia, on the other hand, refers to the connective tissue used to surround and hold together our body’s muscles. As a result, eosinophilic infiltration (increased presence of eosinophils) can act as a useful indicator for eosinophilic fasciitis, also known as Shulman’s syndrome, but the reality is that this infiltration is only seen in approximately half of all investigated case.4
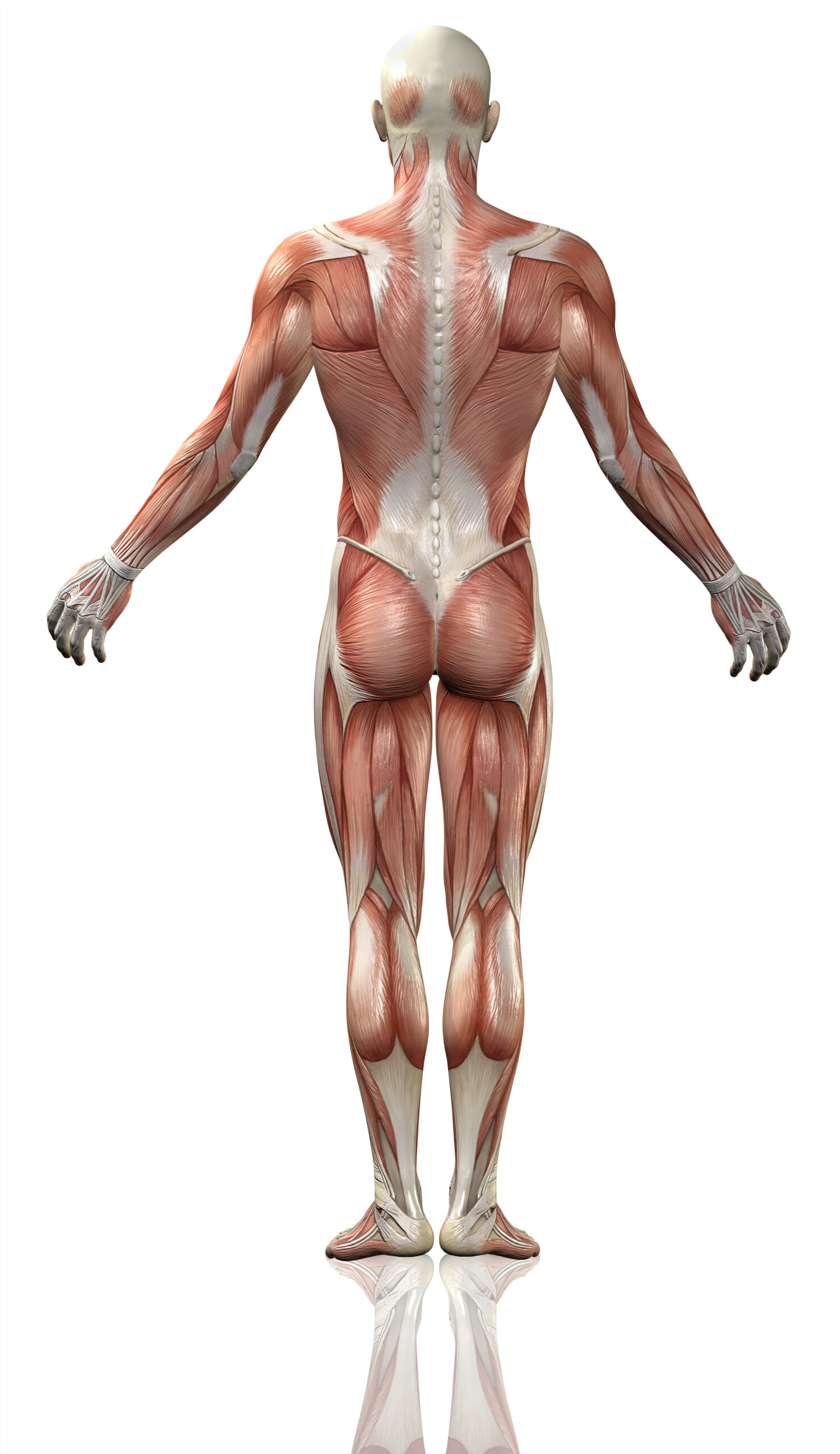
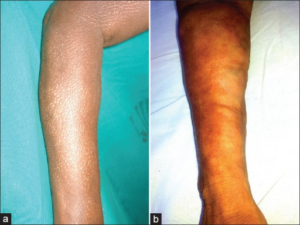
Though the disease is called eosinophilic fasciitis, approximately half of all instances in which eosinophilic infiltration aren’t observed demands additional criteria to be used for diagnosis. To obtain a definitive diagnosis of Shulman’s syndrome, there are two separate pieces of criteria that must be fulfilled according to the Journal of Dermatology. One required piece of criteria includes the display of hardened, scar-like tissue on the four limbs.5 In the context of eosinophilic fasciitis, this refers to the thickening of the skin on the four limbs – excluding fingers and face.6 In addition, at least one minor criterion must be met. This can either take the form of a skin biopsy which displays cellular infiltration of eosinophils and monocytes with visible thickening of the fascia, or the use of magnetic resonance imaging to similarly visualize the thickening of the fascia.7 As a result of the thickened fascia, patients experience the common symptoms associated with eosinophilic fasciitis.
Physically, the thickening of tissue results in hindered limb movement and physical signs used to identify Shulman’s disease.8,9 As mentioned, the thickening of tissue results in limited mobility caused by a flexed joint.10 It is likely that as fascia thickens, this places additional strain on the joint that prevents it from being released from a flexed state. Aside from having a limited limb range of motion, the limbs themselves also present additional characteristics typically expressed in patients. This includes the presence of ‘peau d`orange’ skin and ‘groove signs’, respectively meaning orange-peel skin and the depressions left on the affected limbs.11 It is important to note, however, that there is also a similar disease which exhibits thickening of the skin as well – localized scleroderma.
In considering a diagnosis for the patient, localized scleroderma (also known as morphea) is a likely candidate that can be used to explain diseased individuals with Shulman’s disease. The reason for this is because this disease mimics localized scleroderma in its scarring pattern, which complicates the differentiation between the two.12 Additionally, in rare cases, it can also be possible for morphea and eosinophilic fasciitis to simultaneously be present in a patient.13 With attention to detail, however, it becomes possible to differentiate the two diseases from each other.
To differentiate between the two, it is often helpful to consider not only visible physical differences between the expression of the diseases but also to consider microscopic differences. There is a higher tendency for eosinophilic fasciitis to appear as swelling on both arms or legs while localized scleroderma (morphea) usually presents itself as swelling on only one limb.14 Additionally, morphea also tends to exhibit symptoms which result in loss of blood flow to the extremities (like fingers) and has the potential to cause more serious internal organ damage to the lungs and heart, unlike Shulman’s disease which is usually limited to affecting the skin.15 These can’t be the only metrics used to diagnose patients, however, as there have been recorded cases of individuals with Shulman’s disease only expressing this welling on one limb, and swelling from morphea appearing on both limbs.16 It is for this reason that the previously mentioned biopsy is required to rule out scleroderma since thickening of the tissue in eosinophilic fasciitis is expressed through thickening of the fascia, while morphea usually involves thickening of the dermis.17
So, how would this thickened fascia be treated today? The treatments currently available for treatments of eosinophilic fasciitis vary from physical therapy to chemical treatment for the disease. In most instances, steroids are used as a first-line treatment to improve skin condition.18 When ineffective, second-line treatments can include the use of immunosuppressive agents (used to suppress the immune system’s response), and there are also antibody treatments available for individuals who fail to respond to either first- or second-line treatments.19 Of course, some treatments may be expensive to employ as is the case for antibody treatment. Instead, patients may choose to opt for physical therapy to regain limb mobility. In this case, a combination of steroid treatment and physical therapy can help ease the effects of Shulman’s disease until limb mobility is restored.20 Knowing all this information regarding the symptoms displayed, classifications used to differentiate eosinophilic fasciitis and even potential treatments that can be employed, one would expect that the cause of this disease would be known. Unfortunately, this assumption would be incorrect.
Despite being able to treat the disease, we are still unable to definitively understand the mechanisms responsible for the development of this disease. Most cases of eosinophilic fasciitis continue to be considered idiopathic – they arise spontaneously without a known cause.21 There have been attempts to list potential triggers like strenuous exercise, graft-vs host disease (in which the immune system of transplanted cells attacks the host), and even bacterial infections.22 All of these listed triggers are done on the basis of previously existing studies – most of which are case studies. The fact that they are case studies means it is possible, but unlikely, that these triggers can be seriously considered given the possibility of each being an outlier among any potential larger-scale study. However, there is good reason to believe there is some sort of mechanism within the autoimmune system responsible for aiding in the development of Shulman’s disease based on lab observations of the disease and its known response to steroids in reducing the inflammatory response.23
While the exact mechanism behind the cause of Shulman’s disease remains unknown, some promising hypotheses regarding the causative factors for its appearance have been proposed. There are some claims that the excessive fibrosis, or the thickening of tissue, could be due to increased activity within dermal fibroblasts and other proteins, as well as elevated levels of TIMP-1 (tissue inhibitor of metalloproteinase-1).24 Dermal fibroblasts, specifically reticular fibroblasts, are cells responsible for healing wounds – creating scar tissue in the process.25 In general, the fibroblast family is responsible for creating new connective tissue. An overexpression of these fibroblasts could lead to the excess formation of scar tissue, in which case it makes sense to believe they could lead to thickened fascia as more scar tissue is developed when not needed. As for why TIMP-1 could be involved in the development of eosinophilic fasciitis, this would be due to its relationship with matrix metalloproteinase-1 (MMP-1). It has been noted that the presence of fibrosis exhibits increased TIMP1 expression, which in turn prevents the expression of MMP1 which is responsible for the breakdown of fibrous collagen.26 While this study focused on pituitary adenoma fibrosis, it also mentions how specifically inhibiting the expression of TIMP-1 can also help in treating other fibrotic diseases, which could help against fibrosis in eosinophilic fasciitis.27 While knowing that TIMP-1 can be involved in the increased levels of fibroblasts, it’s also important to note that there are other potential causes that must be considered as well.
In particular, one must also consider the roles that interleukins may have in the development of Shulman’s syndrome. The role of interleukins is to regulate the body’s response in the event of there being an immune response – this also includes any inflammation that may occur.28 Eosinophilic fasciitis is known to be correlated to high levels of interleukin-5 (IL-5), which is known to have a role in increasing the number of eosinophils present in our body.29 This becomes relevant in discussing how this disease develops (pathogenesis) given the presence of eosinophils. While findings indicate that there have been regular instances in which eosinophilic infiltration is not observed, this doesn’t discount the possibility of eosinophils being involved in the formation of this disease. The lack of observed eosinophilic infiltration in some cases can potentially be explained by differing times of diagnosis since this observation only appears early in the formation of the disease.30 This opens the possibility for there being additional cases with undiagnosed excess levels of eosinophils, making an analysis of their effects worthwhile. Especially when considering how eosinophils have been shown to promote the production of interleukins as well as other growth factors that have been shown to stimulate fibroblasts.31
It is imperative that additional research be conducted into eosinophilic fasciitis to provide a clearer understanding of its development for future treatments. Given the low number of reported cases, it is understandable why there may be a lack of research, either due to the lack of patients available for studies or disinterest in further research. However, further research into the disease wouldn’t simply be a fruitless endeavor. It could pave the way for studies into alternative treatments capable of helping individuals who display resistance to first-line and second-line treatments. While not extensively studied, a promising candidate, tocilizumab, has displayed significant effectiveness of treating the disease based on a small sample of six recorded cases.32 It acts as an antibody that works to reduce our body’s inflammatory response by targeting interleukin-6, which is known to stimulate inflammation.33 By considering other targets that are known to stimulate fibroblasts, it is possible that alternative and, more importantly, specific treatments can be made. Besides the help said research would provide for discovering additional treatments, research into this disease may also help better understand other more common diseases.
As mentioned, localized scleroderma (morphea) is a more common disease that is known to display similar symptoms to Shulman’s disease. Given that they both result in similar symptoms of limited limb mobility and appear as thickened tissue, it may be of interest to study eosinophilic fasciitis as a comparative disease to which morphea can be compared to. Alternatively, the mechanisms underlying its development could also provide additional information into autoimmune diseases, particularly those that involve eosinophils. Further exploration into the involvement of eosinophils and bodily conditions, such as expression levels for interleukins or growth factors exhibited in Shulman’s disease can reveal new information applicable to other diseases or conditions that involve similar pathways. Of course, before such applications could be possible, it must first be confirmed if their involvement is required across all cases of eosinophilic fasciitis. Depending on what information is collected, not only patients of this rare disease but also the livelihoods of many others may be impacted by this additional research.
- Aun, J. A., Knox, R. S., & Roberts, J. E. (2021). The enigmatic fascia: Eosinophilic fasciitis. Journal of Osteopathic Medicine, 121(6), 555–559. https://doi.org/10.1515/jom-2020-0284. ↵
- Ihn, H. (2019). Eosinophilic fasciitis: From pathophysiology to treatment. Allergology International, 68(4), 437–439. https://doi.org/10.1016/j.alit.2019.03.001. ↵
- Lombardi, C., Berti, A., & Cottini, M. (2022). The emerging roles of eosinophils: Implications for the targeted treatment of eosinophilic-associated inflammatory conditions. Current Research in Immunology, 3, 42–53. https://doi.org/10.1016/j.crimmu.2022.03.002. ↵
- Ihn, H. (2019). Eosinophilic fasciitis: From pathophysiology to treatment. Allergology International, 68(4), 437–439. https://doi.org/10.1016/j.alit.2019.03.001. ↵
- Jinnin, M., Yamamoto, T., Asano, Y., Ishikawa, O., Sato, S., Takehara, K., Hasegawa, M., Fujimoto, M., & Ihn, H. (2018). Diagnostic criteria, severity classification and guidelines of eosinophilic fasciitis. The Journal of Dermatology, 45(8), 881–890. https://doi.org/10.1111/1346-8138.14160. ↵
- Ihn, H. (2019). Eosinophilic fasciitis: From pathophysiology to treatment. Allergology International, 68(4), 437–439. https://doi.org/10.1016/j.alit.2019.03.001. ↵
- Jinnin, M., Yamamoto, T., Asano, Y., Ishikawa, O., Sato, S., Takehara, K., Hasegawa, M., Fujimoto, M., & Ihn, H. (2018). Diagnostic criteria, severity classification and guidelines of eosinophilic fasciitis. The Journal of Dermatology, 45(8), 881–890. https://doi.org/10.1111/1346-8138.14160. ↵
- El Iskandarani, S., Khasho, M., & Merashli, M. (2023). Tocilizumab in the Treatment of Eosinophilic Fasciitis: A Case Study and Review of Literature. Mediterranean Journal of Rheumatology, 34(1), 78–85. https://doi.org/10.31138/mjr.34.1.78. ↵
- Ihn, H. (2019). Eosinophilic fasciitis: From pathophysiology to treatment. Allergology International, 68(4), 437–439. https://doi.org/10.1016/j.alit.2019.03.001. ↵
- Ihn, H. (2019). Eosinophilic fasciitis: From pathophysiology to treatment. Allergology International, 68(4), 437–439. https://doi.org/10.1016/j.alit.2019.03.001. ↵
- El Iskandarani, S., Khasho, M., & Merashli, M. (2023). Tocilizumab in the Treatment of Eosinophilic Fasciitis: A Case Study and Review of Literature. Mediterranean Journal of Rheumatology, 34(1), 78–85. https://doi.org/10.31138/mjr.34.1.78. ↵
- El Iskandarani, S., Khasho, M., & Merashli, M. (2023). Tocilizumab in the Treatment of Eosinophilic Fasciitis: A Case Study and Review of Literature. Mediterranean Journal of Rheumatology, 34(1), 78–85. https://doi.org/10.31138/mjr.34.1.78. ↵
- Ihn, H. (2019). Eosinophilic fasciitis: From pathophysiology to treatment. Allergology International, 68(4), 437–439. https://doi.org/10.1016/j.alit.2019.03.001. ↵
- Ihn, H. (2019). Eosinophilic fasciitis: From pathophysiology to treatment. Allergology International, 68(4), 437–439. https://doi.org/10.1016/j.alit.2019.03.001. ↵
- Mazilu, D., Boltașiu (Tătaru), L. A., Mardale, D.-A., Bijă, M. S., Ismail, S., Zanfir, V., Negoi, F., & Balanescu, A. R. (2023). Eosinophilic Fasciitis: Current and Remaining Challenges. International Journal of Molecular Sciences, 24(3), 1982. https://doi.org/10.3390/ijms24031982. ↵
- Aun, J. A., Knox, R. S., & Roberts, J. E. (2021). The enigmatic fascia: Eosinophilic fasciitis. Journal of Osteopathic Medicine, 121(6), 555–559. https://doi.org/10.1515/jowh39. https://doi.org/10.1016/j.alit.2019.03.001. ↵
- El Iskandarani, S., Khasho, M., & Merashli, M. (2023). Tocilizumab in the Treatment of Eosinophilic Fasciitis: A Case Study and Review of Literature. Mediterranean Journal of Rheumatology, 34(1), 78–85. https://doi.org/10.31138/mjr.34.1.78. ↵
- El Iskandarani, S., Khasho, M., & Merashli, M. (2023). Tocilizumab in the Treatment of Eosinophilic Fasciitis: A Case Study and Review of Literature. Mediterranean Journal of Rheumatology, 34(1), 78–85. https://doi.org/10.31138/mjr.34.1.78. ↵
- El Iskandarani, S., Khasho, M., & Merashli, M. (2023). Tocilizumab in the Treatment of Eosinophilic Fasciitis: A Case Study and Review of Literature. Mediterranean Journal of Rheumatology, 34(1), 78–85. https://doi.org/10.31138/mjr.34.1.78. ↵
- Aun, J. A., Knox, R. S., & Roberts, J. E. (2021). The enigmatic fascia: Eosinophilic fasciitis. Journal of Osteopathic Medicine, 121(6), 555–559. https://doi.org/10.1515/jom-2020-0284. ↵
- Mazilu, D., Boltașiu (Tătaru), L. A., Mardale, D.-A., Bijă, M. S., Ismail, S., Zanfir, V., Negoi, F., & Balanescu, A. R. (2023). Eosinophilic Fasciitis: Current and Remaining Challenges. International Journal of Molecular Sciences, 24(3), 1982. https://doi.org/10.3390/ijms24031982. ↵
- Mazilu, D., Boltașiu (Tătaru), L. A., Mardale, D.-A., Bijă, M. S., Ismail, S., Zanfir, V., Negoi, F., & Balanescu, A. R. (2023). Eosinophilic Fasciitis: Current and Remaining Challenges. International Journal of Molecular Sciences, 24(3), 1982. https://doi.org/10.3390/ijms24031982. ↵
- Mazilu, D., Boltașiu (Tătaru), L. A., Mardale, D.-A., Bijă, M. S., Ismail, S., Zanfir, V., Negoi, F., & Balanescu, A. R. (2023). Eosinophilic Fasciitis: Current and Remaining Challenges. International Journal of Molecular Sciences, 24(3), 1982. https://doi.org/10.3390/ijms24031982. ↵
- El Iskandarani, S., Khasho, M., & Merashli, M. (2023). Tocilizumab in the Treatment of Eosinophilic Fasciitis: A Case Study and Review of Literature. Mediterranean Journal of Rheumatology, 34(1), 78–85. https://doi.org/10.31138/mjr.34.1.78. ↵
- Hannen, R., Connelly, J., Myers, S., & Ojeh, N. (2023). Chapter 15—Skin tissue engineering and keratinocyte stem cell therapy. In J. De Boer, C. A. V. Blitterswijk, J. A. Uquillas, & N. Malik (Eds.), Tissue Engineering (Third Edition) (pp. 491–532). Academic Press. https://doi.org/10.1016/B978-0-12-824459-3.00031-7. ↵
- Wang, H., Li, W.-S., Shi, D.-J., Ye, Z.-P., Tai, F., He, H.-Y., Liang, C.-F., Gong, J., & Guo, Y. (2008). Correlation of MMP1 and TIMP1 expression with pituitary adenoma fibrosis. Journal of Neuro-Oncology, 90(2), 151–156. https://doi.org/10.1007/s11060-008-9647-9. ↵
- Wang, H., Li, W.-S., Shi, D.-J., Ye, Z.-P., Tai, F., He, H.-Y., Liang, C.-F., Gong, J., & Guo, Y. (2008). Correlation of MMP1 and TIMP1 expression with pituitary adenoma fibrosis. Journal of Neuro-Oncology, 90(2), 151–156. https://doi.org/10.1007/s11060-008-9647-9. ↵
- Justiz Vaillant, A. A., & Qurie, A. (2023). Interleukin. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK499840/. ↵
- Justiz Vaillant, A. A., & Qurie, A. (2023). Interleukin. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK499840/. ↵
- Ihn, H. (2019). Eosinophilic fasciitis: From pathophysiology to treatment. Allergology International, 68(4), 437–439. https://doi.org/10.1016/j.alit.2019.03.001. ↵
- El Iskandarani, S., Khasho, M., & Merashli, M. (2023). Tocilizumab in the Treatment of Eosinophilic Fasciitis: A Case Study and Review of Literature. Mediterranean Journal of Rheumatology, 34(1), 78–85. https://doi.org/10.31138/mjr.34.1.78. ↵
- El Iskandarani, S., Khasho, M., & Merashli, M. (2023). Tocilizumab in the Treatment of Eosinophilic Fasciitis: A Case Study and Review of Literature. Mediterranean Journal of Rheumatology, 34(1), 78–85. https://doi.org/10.31138/mjr.34.1.78. ↵
- El Iskandarani, S., Khasho, M., & Merashli, M. (2023). Tocilizumab in the Treatment of Eosinophilic Fasciitis: A Case Study and Review of Literature. Mediterranean Journal of Rheumatology, 34(1), 78–85. https://doi.org/10.31138/mjr.34.1.78. ↵