In March of 2017, Trulize Hernandez worked ferociously in the garden on a bright Sunday morning. The dry El Paso air was spring with dust storms and pollen, typical for the desert climate that she had become so accustomed to in the years prior. Though, it was not out of the ordinary, Hernandez woke up the next morning to find that her voice had vanished almost completely. At that exact moment, it was nothing urgent, no life-or-death matter that occupied her mind. However, as she looked back on her life, she began to recount periods in her life which she now refers to as flare-ups. Spells of extreme dizziness seemed to plague her life, sending her down a whirlwind of unsteadiness. Even the smallest exposure to heat resulted in profuse amounts of sweating, which in turn made Hernandez feel weak all throughout her body. Weak to the point where even walking up the stairs became too much to handle. Then, almost instantaneously, the spells of exhaustion and pain would cease to exist. This particular Monday, however, proved to be much different than the rest. For the next year, Hernandez’s voice would remain diminished, among other more severe symptoms that would continue to slowly present themselves, accumulating and making her life exceedingly more difficult as time progressed.
The extreme exhaustion that once plagued Hernandez in her younger years reappeared once again, this time to the point where driving home from work seemed like she was making an hours long commute. Her body desired to shut off. Then, severe headaches began to manifest. Not long after this, her temples began to swell profusely, and her lungs grew weaker and weaker by the day. This caught the attention of her physician, who immediately suggested that she undergo an emergency MRI out of fear that she might have a developing brain tumor. Upon receiving an MRI, the lab technician grew concerned at the sight of her results. The results? An MRI image that showed what looked to be like the brain of a 90-year-old rather than that of a 40-year-old. Lesions enveloped her brain and the doctors explained that it could be only because of two reasons. The first being the occurrence of multiple strokes not typical of someone her age. The second being the ongoing development of a demyelinating disease known as multiple sclerosis. Following this initial suspicion, Hernandez was immediately referred to a neurologist where she underwent a lumbar puncture and had spinal fluid retrieved in order to accurately diagnose her. To no surprise, Hernandez’s antinuclear antibody test came back positive for multiple sclerosis. Shock began to set in as she realized her battle with this peculiar disease had just begun.

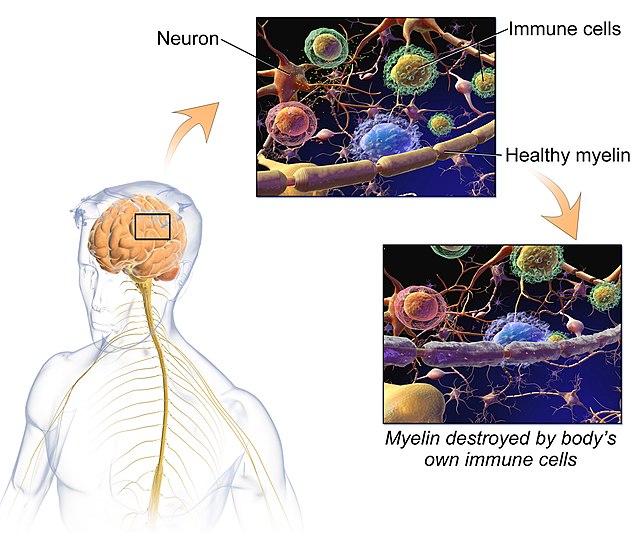
To much of the nation’s population, multiple sclerosis is a rather unheard-of disease whose mechanisms are not in our repertoire of common knowledge. Multiple sclerosis is a demyelinating disease in which the myelin surrounding the nerve fibers in our central and peripheral nervous systems are irreversibly damaged and degraded. Myelin is made by oligodendrocytes in the central nervous system (CNS) and Schwann cells in the peripheral nervous system (PNS). The two different compositions of myelin differentiate the two in several ways. One oligodendrocyte forms the myelin sheath for several neurons, yet a single Schwann cell myelinates only one segment for one neuron. During the development of the vertebrate nervous system, myelination starts around birth first in the PNS, then the spinal cord and finally in the brain. Most myelination is completed in the PNS shortly after birth, but myelination in the CNS is an ongoing process that continues throughout adulthood.1 The myelin sheath serves to insulate the axons of nerves and in turn, allows for the undisrupted conduction of nerve impulses to and from the brain.2 As a result of the myelin sheath’s destruction, brain, spinal cord, and optic pathway damage present themselves in the near 2.5 million people affected by the disease globally. There is no one sure factor that contributes to the development of the disease, rather there are a conglomerate of factors that contribute to MS pathogenesis such as genetics, environmental factors such as sunlight and vitamin D, smoking, or the Epstein-Barr virus. In the case of genetics, one of the main risks for MS, especially in Hispanics and African Americans, lies in the HLA-DRB1*1501 allele.3 Heterozygotes for this allele have an odds ratio of MS>3 and homozygotes >6. And through many genome-wide association studies, over 150 single nucleotide polymorphisms that are associated with MS susceptibility have been identified, most of them located close to genes that help code for processes such as proper immune function.4
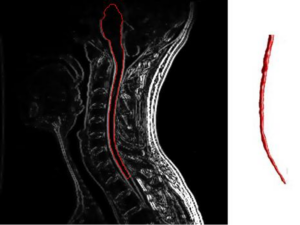
Before diagnosis, a person is typically suspected of having the disease when they present a clinically isolated syndrome in which lesions present themselves on locations such as the brainstem and spinal cord. Multiple sclerosis will also typically present itself through two events: inflammatory flare-ups and disability progression.5 The disease also presents itself in two main progressive types: relapsing forms and progressive forms. Relapsing forms are the most frequent of the two and can be diagnosed by the appearance of inflammatory attacks that lead to a progression of disability. Relapsing forms of multiple sclerosis preferentially affect women as well, and after approximately 10 years can further progress to a secondary progressive form. On the contrary, primary progressive forms of the disease are characterized by progressive and irreversible courses without any periods of calm in between.6 Relapses in multiple sclerosis can develop over the course of a few hours to several days. They usually level off at an intensity that can last up to several weeks before eventually recovering, and though it may seem as though the relapse has subsided completely, there may be residual damage left from the attack such as loss of color vision or the appearance of depth perception abnormalities. Over time, recurring relapses can in turn lead to sustained disability in a patient.7
Upon receiving her diagnosis, Hernandez explained that the pure shock of receiving her diagnosis was already enough to handle. Now, she had to learn how to cope with this news while also becoming acclimated to the new string of treatments that doctors would introduce to her in hopes of slowing the progression of the disease. Immediately, Hernandez was put on 40 milligram injections of Copaxone, directed to be administered three times a week. The hope was that through these injections, the number of relapses that she was experiencing would eventually decrease. Rather than bring the disease to a halt though, the injections instead brought with them ravaging side effects that left lasting damage for years to come. Skin damage at the site of infections was common, as Hernandez developed painful hives and bruises across the entirety of her stomach. Demyelination of the nerves is often secondary to infectious disease, ischemic disease, metabolic disorders, or hereditary disorders.8 This proved to be true with Hernandez as the presence of Dermatomyositis and Scleroderma was detected during a severe MS flare-up in early 2018. Hernandez went to bed one night with extreme pain, weakness, and a rash, only to wake up and find that it was almost impossible to walk out of her room on her own. Doctors explained that the two newfound diseases were overlapping connective tissue diseases in which the immune system attacked the skin and muscles much like her MS had been doing previously. As a result, her neurologist immediately ordered 1000mg of a Solu-Medrol IV once a day for 3 days while she was held in the hospital. A Solu-Medrol IV is also known as Intravenous Methylprednisolone. It is a drug similar to prednisone but can typically be administered at higher doses as an infusion for treatment of severe inflammation and flare-ups. By the 3rd dose of the Solu-Medrol, Hernandez was back on her feet and walking out of the hospital on her own.
Though the Solu-Medrol proved to work for the time being, another major flare-up would present itself in September of that same year. This time, the cause of the flare-up was Hernandez’s newly diagnosed rheumatoid arthritis combined with her multiple sclerosis. Once again, she was put on Solu-Medrol and Copaxone. However, this time around was different as she did not immediately regain the ability to walk on her own. It was then that it was decided she would receive Rituxin infusions, a drug that helps combat rheumatoid arthritis as well as multiple sclerosis, once every 6 months. These infusions left her with severe headaches, nausea, and feelings of malaise for at least two weeks after every infusion. Needless to say, the drugs that were supposed to alleviate the symptoms of Hernandez’s newfound disease were in fact compounding her feelings of unease and illness.
Treatment for multiple sclerosis can be divided into three separate categories: acute relapse management, disease-modifying treatments (DMTs), and symptomatic treatments. The priority in acute relapse management is to treat any associated infections such as a urinary tract infection, which itself can cause a true relapse. The difficulty with managing relapses lies in the fact that it can be a true relapse or a fluctuation due to an existing demyelinating lesion.9 If the relapse is of moderate functional severity or worse, then doctors will often prescribe high-dose methylprednisolone therapy at doses ranging from 500 to 1000 milligrams per day for 3 to 5 days. A COPOUSEP trial actually demonstrated that oral administration of the drug was not inferior to intravenous methylprednisolone given that the dose is high enough.10
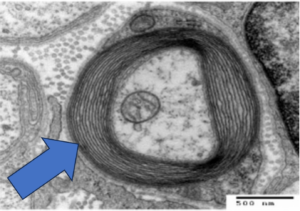
Disease-modifying treatment is somewhat of a revolution in the treatment of multiple sclerosis. One disease-modifying treatment that has been approved on a global scale for the treatment of relapsing multiple sclerosis is Copaxone, also known by its generic name glatiramer acetate. It is a synthetic copolymer with an amino acid composition based on the structure of myelin basic protein, one of the autoantigens involved in the pathogenesis of the disease.11 Initially, it was developed as a tool to study experimental autoimmune encephalomyelitis but instead unexpectedly inhibited disease and was developed as treatment for MS as a result. Copaxone significantly reduces relapse rate and progression of disability in MS by induction of glatiramer-reactive regulatory cells including CD4+ and CD8+ T-cells, which can work with myelin antigens to produce anti-inflammatory effects and neuron protection in the brain.12 As previously discussed, the diagnosis of multiple sclerosis comes with an array of disabling symptoms such as fatigue, cognitive impairment, and extreme pain. The James Lind Alliance Priority Setting Partnership has stated that one of the top 10 priorities in the battle against MS is to find treatments that are effective in improving cognition in people with MS.13 Several symptomatic treatments for symptoms such as pain or fatigue have also been suggested and proven to be effective to some degree. The challenge lies in battling these symptoms as the disease continues to inevitably progress and worsen.
Though disease-modifying treatments have been found to significantly reduce the probability of relapses, inhibit the formation of new brain lesions, and reduce exacerbations, many of these treatments also carry a risk of side effects ranging from mild to severe.14 In many patients, it has been suggested that the the likelihood of taking a disease-modifying treatment decreases as the likelihood of severe side effects such as fatal brain infections increases. When discussing these treatments and their side effects, it is important to discuss both the benefits and risks of treatment in a supportive environment in order to improve the likelihood of a patient remaining committed to a treatment for multiple sclerosis. Hope lies in other forms of therapy and treatment, such is the case with CAR T-cell therapy. CAR T-cell therapy involves collecting the T-cells of a patient’s immune system, whose function is to activate immune responses within the body to kill infected cells and tumor cells directly. The T-cells are modified so that they are programmed to attack new targets, such as specific cancer cells, when infused back into the body. The therapy has been used to effectively combat autoimmune diseases such as lupus, along with various types of cancer and it is suspected that one day the therapy can be implemented into treatment regimens for multiple sclerosis as well.15
Nearly 5 years removed from her diagnosis with multiple sclerosis, Hernandez has gradually learned how to cope with the disease and manage her lifestyle in a way that supports her treatment regime. After treatments at the Mayo Clinic in Arizona just over a year ago, she states that the combination of medications such as Copaxone and Solu-Medrol along with continued rest and the avoidance of stress has helped her avoid a flare-up in that time span. Once the Director of Pharmacy for a community health center with multiple pharmacies, Hernandez was forced to retire medically as she stated that she had to consider her family and work on living for them. The psychological effect of the disease is immense, but she refused to let herself get pulled down by it because of her 5 children and husband that depended on her being happy, cheerful, and full of life. Hernandez was determined to prevent her disease from becoming theirs as well. She kept their quality of life at the forefront of her mind and was determined to do anything it took to stay as healthy as possible for their sakes. As is for nearly all patients diagnosed with multiple sclerosis, Hernandez has been left with permanent disabilities that will remain with her for the rest of her life, and she has come to recognize that she will never be able to return to her old self again. However, she is grateful for the chance to even continue this game of life in the first place.
Multiple sclerosis is a truly debilitating disease. Patients diagnosed with the disease survive in a bubble in which everything has to be perfectly fitted and adjusted in order to accompany for the symptoms and flare-ups that they experience. But in many ways, it also serves as a reminder as to how precious life can be, as we often tend to take ordinary moments in our day-to-day routines for granted. Hernandez’s outlook on life is simple. Do not allow yourself to think about what the future holds, and do not dwell on what you do not have or what you can no longer do. Focus on what you do have and make the best of it. She credits her husband for adjusting to her newfound lifestyle and providing for her in a manner that makes the disease just a bit easier to live with. And she plans to step into everyday with the same positive mindset that has helped her battle multiple sclerosis for the past 5 years, refusing to let this disease define who she is and how she lives her life.
- Poitelon, Y., Kopec, A. M., & Belin, S. (2020). Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells, 9(4), 812. https://doi.org/10.3390/cells9040812. ↵
- Ortiz, G. G., Torres-Mendoza, B. M. G., Ramírez-Jirano, J., Marquez-Pedroza, J., Hernández-Cruz, J. J., Mireles-Ramirez, M. A., Torres-Sánchez, E. D., (2023). Genetic Basis of Inflammatory Demyelinating Diseases of the Central Nervous System: Multiple Sclerosis and Neuromyelitis Optica Spectrum. Genes, 14(7), 1319. https://doi.org/10.3390/genes14071319. ↵
- Beecham, A. H., Amezcua, L., Chinea, A., Manrique, C. P., Gomez, L., Martinez, A., Beecham, G. W., Patsopoulos, N. A., Chitnis, T., Weiner, H. L., De Jager, P. L., Burchard, E. G., Lund, B. T., Fitzgerald, K. C., Calabresi, P. A., Delgado, S. R., Oksenberg, J. R., (2022). Ancestral risk modification for multiple sclerosis susceptibility detected across the Major Histocompatibility Complex in a multi-ethnic population. PLoS One, 17(12), e0279132. https://doi.org/10.1371/journal.pone.0279132. ↵
- Dobson, R., & Giovannoni, G. (2019). Multiple sclerosis—A review. European Journal of Neurology, 26(1), 27–40. https://doi.org/10.1111/ene.1381. ↵
- Ortiz, G. G., Torres-Mendoza, B. M. G., Ramírez-Jirano, J., Marquez-Pedroza, J., Hernández-Cruz, J. J., Mireles-Ramirez, M. A., Torres-Sánchez, E. D., (2023). Genetic Basis of Inflammatory Demyelinating Diseases of the Central Nervous System: Multiple Sclerosis and Neuromyelitis Optica Spectrum. Genes, 14(7), 1319. https://doi.org/10.3390/genes14071319. ↵
- Ortiz, G. G., Torres-Mendoza, B. M. G., Ramírez-Jirano, J., Marquez-Pedroza, J., Hernández-Cruz, J. J., Mireles-Ramirez, M. A., Torres-Sánchez, E. D., (2023). Genetic Basis of Inflammatory Demyelinating Diseases of the Central Nervous System: Multiple Sclerosis and Neuromyelitis Optica Spectrum. Genes, 14(7), 1319. https://doi.org/10.3390/genes14071319. ↵
- Dobson, R., & Giovannoni, G. (2019). Multiple sclerosis—A review. European Journal of Neurology, 26(1), 27–40. https://doi.org/10.1111/ene.1381. ↵
- Ortiz, G. G., Torres-Mendoza, B. M. G., Ramírez-Jirano, J., Marquez-Pedroza, J., Hernández-Cruz, J. J., Mireles-Ramirez, M. A., Torres-Sánchez, E. D., (2023). Genetic Basis of Inflammatory Demyelinating Diseases of the Central Nervous System: Multiple Sclerosis and Neuromyelitis Optica Spectrum. Genes, 14(7), 1319. https://doi.org/10.3390/genes14071319. ↵
- Doshi, A., & Chataway, J. (2016). Multiple sclerosis, a treatable disease. Clinical Medicine (London, England), 16(Suppl 6), s53–s59. https://doi.org/10.7861/clinmedicine.16-6-s53. ↵
- Doshi, A., & Chataway, J. (2016). Multiple sclerosis, a treatable disease. Clinical Medicine (London, England), 16(Suppl 6), s53–s59. https://doi.org/10.7861/clinmedicine.16-6-s53. ↵
- Dhib-Jalbut, S. (2003). Glatiramer acetate (Copaxone®) therapy for multiple sclerosis. Pharmacology & Therapeutics, 98(2), 245–255. https://doi.org/10.1016/S0163-7258(03)00036-6. ↵
- Dhib-Jalbut, S. (2003). Glatiramer acetate (Copaxone®) therapy for multiple sclerosis. Pharmacology & Therapeutics, 98(2), 245–255. https://doi.org/10.1016/S0163-7258(03)00036-6. ↵
- Doshi, A., & Chataway, J. (2016). Multiple sclerosis, a treatable disease. Clinical Medicine (London, England), 16(Suppl 6), s53–s59. https://doi.org/10.7861/clinmedicine.16-6-s53. ↵
- Jarmolowicz, D. P., Bruce, A. S., Glusman, M., Lim, S.-L., Lynch, S., Thelen, J., Catley, D., Zieber, N., Reed, D. D., & Bruce, J. M. (2017). On how patients with multiple sclerosis weigh side effect severity and treatment efficacy when making treatment decisions. Experimental and Clinical Psychopharmacology, 25(6), 479–484. https://doi.org/10.1037/pha0000152. ↵
- Scientists Hail Autoimmune Disease Therapy Breakthrough; Study Finds CAR T-Cell Treatment Sends Lupus into Remission, Raising Hopes It Could Be Used to Treat Diseases Such Multiple Sclerosis. – Document – Gale General OneFile, n.d. ↵