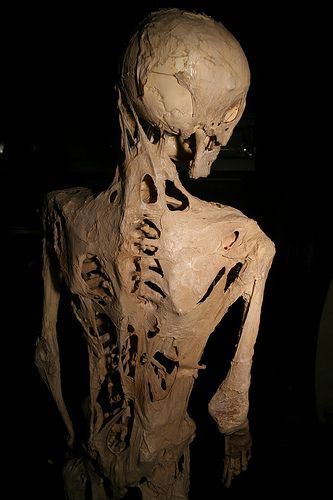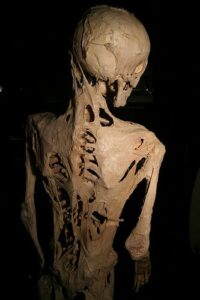
Harry Raymond Eastlack Jr. is among the most famous cases of someone being diagnosed and having an incredibly debilitating and rare disease known as Fibrodysplasia Ossificans Progressiva (FOP).1 Born in 1933, Harry would not have any noticeable symptoms until later in life after having broken his femur in 1937 and then being diagnosed with FOP the next year. As time went on, his arms and legs slowly locked into place as his bones and spine began to fuse together. In 1958 he was admitted into a long-term care facility, where he lived out the rest of his life in pain and forced to face a harsh truth. Near the end of his life, he was only able to move his lips and asked for his skeleton to be donated to science to understand his affliction. Since then, we have come to understand more about the disease, its primary cause, and how it works within the body.
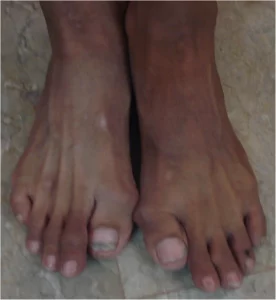
Affecting one person in every two million people, it is incredibly rare to come across this disease, and it has caused the people who are affected by it to rarely, if ever, find someone else with the condition.2 Jeannie Peeper was born in 1958 and was noted to have two crooked big toes and a lump growing on the back of her head, which disappeared as quickly as it was noticed. After her parents noticed that her mouth would not open as wide as usual, they took her to various doctors to look for a diagnosis, and at the age of four, she was diagnosed with FOP.3 In 1999, another girl, Jasmine Floyd, a kindergartener at the time, was diagnosed with FOP after she developed small lumps over her body that quickly disappeared along with a neck that slowly stiffened to the point that she began to lose the ability to turn her head around.4
FOP typically causes the growth of bone by replacing already existing muscle cells in the body after trauma to the area.2 This causes symptoms such as growing pains near joints, stiffness of joints, loss of mobility after bruising or trauma, and swelling nearby joints or sites of trauma. These are all side effects of the growth of bone in places where it should not be caused by FOP. Peeper was able to play around outside freely and without care as a child until one day she realized her arm was stuck in place and she could not dress herself. After being taken in for a biopsy to test the tissue Peeper had to wear a cast, and after 6 months, her arm was completely locked in place. Floyd would remember waking up and being unable to move another joint along with an intense pain that accompanied the freezing of another joint. Though it affects everyone differently, FOP eventually does enough damage that people who are afflicted with it must use a wheelchair or cannot move at all. Later in life, both women would grow up forced to adapt to the lifestyle imposed onto them by a disease that slowly turned their bodies into a prison.
The excess bone growth that is typically associated with FOP is not just bone growing on top of preexisting cells, but it is the complete replacement of damaged or destroyed muscle cells by replacing them with several types of cells needed to grow bones. Until recently, the mechanism of this was not completely understood, and even now, we do not know the exact mechanism; only the associated genes and proteins responsible. As it turns out people affected by FOP typically have a mutation in their ACVR1 gene, which is responsible for creating a receptor that latches onto a protein known as bone morphogenetic protein 4(BMP4). This mutation causes the gene to hyperactivate and causes BMP4 to be expressed when it should not be used.6
Since FOP is such a rare disease it was very difficult to get first-hand advice on the disease much less find other people who also had FOP, so when the opportunity presented itself, Peeper managed to find and contact several people who also happened to have FOP. What started as mailing letters asking for advice or exchanging experiences would eventually become the International FOP Association.14 As its founding member, Peeper was able to make many connections with others affected by the disease to form support groups for those seeking help in some form or another along with getting into contact with willing researchers who wanted to find the cause of and potential treatments of FOP.
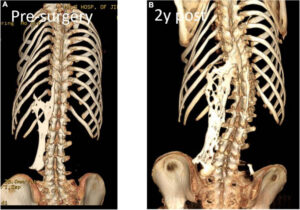
Although research on FOP is still ongoing, a solid treatment to eliminate the effects of FOP has been found yet, which means that those who have FOP still need to be careful after receiving their diagnosis. While flare-ups can be random, they are often caused by trauma which can be caused by a bump or a bruise. Banging your knee on the coffee table could mean you just lost the ability to move that knee. Getting hit by a ball to the chest could mean that it will become harder and harder to breathe as bone grows over your rib cage. Falling could mean you just lost the ability to move on your own without assistance. If you were careful not to bruise or bump into anything it could all, be futile if you were to get an infection that caused swelling, which could cause you to lose mobility wherever it occurred. Even going for a routine check-up by the doctor might cause some trauma later. If you have another condition that requires surgery on the back or somewhere else in the body, you may very well cause a total lock-up of the area. Even if you avoided all kinds of traumas, sickness, and surgeries you could still get a random flare-up and be unable to walk in the morning or open your mouth at all. On top of all these things, each flare-up causes excruciating pain as your body is forced to get used to the bones growing in places where they should not be, and the bone growths rub against each other as they slowly lock up your joints.
The road to understanding FOP has been long and difficult since obtaining the necessary tissue sample for research calls for biopsies, which are harmful enough to someone who already has FOP. Without backing and funding research for FOP would not be anywhere near where it is today. Kaplan would not have been able to lead a team of researchers without the fundraisers done through IFOPA in the hopes of progressing the understanding of the disease and getting closer to a cure.
Through the funding and IFOPA Kaplan and his team were able to successfully determine the correlating protein that caused the bone growth along with the gene that caused the disease in the first place. They had originally come across BMP4 and were trying to find out how it worked in FOP until they by coincidence found ACVR1, the gene responsible for causing the disease and allowing BMP4 to grow more bones.
Through these same funds, they were able to get a better understanding of how the mechanism of the disease works and how different factors come into play to grow bones in places where they do not belong. Additionally, they have been able to find interactions between infections and FOP. Since infections invoke an immune response, macrophages invade the surrounding tissue causing swelling in the area which is treated the same way as the other parts of the body and invokes a flare-up causing more bone growth in the surrounding area. In one case study, a 45-year-old woman with FOP had a mild case of COVID-19 but did not need to go to the hospital. After recovering it was found that she was having several flare-ups near the soft tissue surrounding her neck. In this instance, after recovering from COVID-19 the patient has exacerbated the effects of FOP and the radiotherapy she had been going through to lessen the effects of FOP was not slowing down the progression of the flare-ups at all.16
This research has also yielded some positive results as well and we are slowly getting closer to understanding how to treat FOP and treatments that could be used in the future. Some research has identified a possible signaling pathway for BMP and they are in the process of selecting potential candidates for treating the disease.17 Other research has found that the inhibition of Hif1a has shown results in preventing trauma-induced bone growth or ossification. This an is important finding since HiF1a has been shown to be critical in the formation of cartilage which in FOP is essential for growing extra bone since it uses a cartilage intermediary before forming a solid lump of bone.18
Although a diagnosis of FOP can be confusing, scary, and discouraging, some of the people who have it have learned to live with it and make the most of their lives. On average flare-ups begin to happen around a person’s teenage years and they do not suffer the lock up of their limbs or joints yet. While the intensity of FOP varies from person to person most people afflicted with can go through life with some degree of normality, with some people being able to go all the way through college or even getting married before FOP starts to take away their freedom of movement. Peeper was able to get through college and pursue a degree in social work and eventually founded IFOPA even after FOP took away her ability to walk. But because FOP is different for everyone, not everyone gets to live out their lives in any sense of normality. Floyd was affected by flare-ups and was eventually forced to be bed-bound at around the age of twenty-three, but even before this, she took as many chances as she could explore, travel, and go to concerts to enjoy as many things as she could before being bed-bound.
Being diagnosed with such a rare disease could knock out any sense of hope for the parents of a young child or young adult for the possibility of leading a normal life. Thanks to Peeper’s efforts and the existence of IFOPA, people with the diagnosis can find advice, share stories, and make bonds with other people. Through IFOPA people are also able to contribute to research efforts by volunteering for clinical trials or participating in case studies to better understand the disease and hopefully find a cure of some sort. Even now with all the research efforts going on Peeper has accepted that any cure or treatment is not going to come within her lifetime, but she has hope that through her efforts to raise awareness and fund research, a cure or an effective treatment will eventually be found so that others will not have to suffer from this disease.
- Harry and Carol. (n.d.). Retrieved October 20, 2023, from https://muttermuseum.org/exhibitions/harry-and-carol ↵
- Kamal, A. F., Novriansyah, R., Rahyussalim, Prabowo, Y., & Siregar, N. C. (2015). Fibrodysplasia Ossificans Progressiva: Difficulty in Diagnosis and Management A case report and literature review. Journal of Orthopaedic Case Reports, 5(1), 26–30. https://doi.org/10.13107/jocr.2250-0685.248 ↵
- Zimmer, C. (2013, May 23). The Girl Who Turned to Bone. The Atlantic. https://www.theatlantic.com/magazine/archive/2013/06/the-mystery-of-the-second-skeleton/309305/ ↵
- One Spirit, Two Skeletons. (2022, February 28). One Spirit, Two Skeletons. https://jasminfloyd.com/ ↵
- Kamal, A. F., Novriansyah, R., Rahyussalim, Prabowo, Y., & Siregar, N. C. (2015). Fibrodysplasia Ossificans Progressiva: Difficulty in Diagnosis and Management A case report and literature review. Journal of Orthopaedic Case Reports, 5(1), 26–30. https://doi.org/10.13107/jocr.2250-0685.248 ↵
- Frontiers | Fibrodysplasia Ossificans Progressiva: What Have We Achieved and Where Are We Now? Follow-up to the 2015 Lorentz Workshop. (n.d.). Retrieved October 19, 2023, from https://www.frontiersin.org/articles/10.3389/fendo.2021.732728/full ↵
- Shah, Z. A., Rausch, S., Arif, U., & El Yafawi, B. (2019). Fibrodysplasia ossificans progressiva (stone man syndrome): A case report. Journal of Medical Case Reports, 13(1), 364. https://doi.org/10.1186/s13256-019-2297-z ↵
- Kaplan, F. S., Al Mukaddam, M., Stanley, A., Towler, O. W., & Shore, E. M. (2020). Fibrodysplasia ossificans progressiva (FOP): A disorder of osteochondrogenesis. Bone, 140, 115539. https://doi.org/10.1016/j.bone.2020.115539 ↵
- Pignolo, R. J., Shore, E. M., & Kaplan, F. S. (2013). Fibrodysplasia ossificans progressiva: Diagnosis, management, and therapeutic horizons. Pediatric Endocrinology Reviews: PER, 10 Suppl 2(0 2), 437–448. ↵
- Sun, D., Liu, P., Wang, Z., Mu, J., & Cao, J. (2022). Fibrodysplasia ossificans progressiva: A rare disease with spinal deformity and severe hip dysfunction. Frontiers in Pediatrics, 10, 981372. https://doi.org/10.3389/fped.2022.981372 ↵
- Pan, Y., Jiang, Z., Ye, Y., Zhu, D., Li, N., Yang, G., & Wang, Y. (2023). Role and Mechanism of BMP4 in Regenerative Medicine and Tissue Engineering. Annals of Biomedical Engineering, 51(7), 1374–1389. https://doi.org/10.1007/s10439-023-03173-6 ↵
- Kaplan, F. S., Al Mukaddam, M., Stanley, A., Towler, O. W., & Shore, E. M. (2020). Fibrodysplasia ossificans progressiva (FOP): A disorder of osteochondrogenesis. Bone, 140, 115539. https://doi.org/10.1016/j.bone.2020.115539 ↵
- Dinther, M. van, Visser, N., de Gorter, D. J., Doorn, J., Goumans, M.-J., de Boer, J., & ten Dijke, P. (2010). ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. Journal of Bone and Mineral Research, 25(6), 1208–1215. https://doi.org/10.1359/jbmr.091110 ↵
- IFOPA – International Fibrodysplasia Ossificans Progressiva Association. (n.d.). IFOPA – International Fibrodysplasia Ossificans Progressiva Association. Retrieved October 19, 2023, from https://www.ifopa.org/ ↵
- Shah, Z. A., Rausch, S., Arif, U., & El Yafawi, B. (2019). Fibrodysplasia ossificans progressiva (stone man syndrome): A case report. Journal of Medical Case Reports, 13(1), 364. https://doi.org/10.1186/s13256-019-2297-z ↵
- Grgurevic, L., Novak, R., Hrkac, S., Salai, G., & Grazio, S. (2021). Post-COVID-19 exacerbation of fibrodysplasia ossificans progressiva with multiple flare-ups and extensive heterotopic ossification in a 45-year-old female patient. Rheumatology International, 41(8), 1495–1501. https://doi.org/10.1007/s00296-021-04911-6 ↵
- Meng, X., Wang, H., Hao, J., & Link to external site, this link will open in a new tab. (2022). Recent progress in drug development for fibrodysplasia ossificans progressiva. Molecular and Cellular Biochemistry, 477(10), 2327–2334. https://doi.org/10.1007/s11010-022-04446-9 ↵
- Agarwal, S., Loder, S., Brownley, C., Cholok, D., Mangiavini, L., Li, J., Breuler, C., Sung, H. H., Li, S., Ranganathan, K., Peterson, J., Tompkins, R., Herndon, D., Xiao, W., Jumlongras, D., Olsen, B. R., Davis, T. A., Mishina, Y., Schipani, E., & Levi, B. (2016). Inhibition of Hif1α prevents both trauma-induced and genetic heterotopic ossification. Proceedings of the National Academy of Sciences of the United States of America, 113(3), E338–E347. ↵